Join Our Groups
TOPIC 3: SOIL CHEMISTRY

Soil Formation
Soil Formation
Describe soil formation
Soil is formed by the process of weathering. All types of weathering (physical, chemical or biological) result to disintegration of rocks into smaller particles. Air and water enter the space between these particles and chemical changes take place, which lead to the production of chemical substances. Bacteria and plant life soon appear. When plants and animals die, they decay and produce humus. Bacteria and other decomposers play a vital role in the decomposition of plant and animal substrata. The end product of these mechanical, chemical and biological processes is soil.
Therefore, soil can be defined as unconsolidated mineral (inorganic) and organic material on the immediate surface of the earth‟s crust that serves as the medium for plant growth.
All soils contain mineral matter, organic matter, water, air and living organisms, especially bacteria. If any one of these is substantially reduced in amount or is removed from the soil, then the soil deteriorates. There are many types of soil and each has specific characteristics related to the climate, the vegetation and the rock of the region in which it forms. The weathering processes of a region also play an important part in determining soil characteristics. The relationship of these factors is as shown in figure 3.1.

The Factors Influencing Soil Formation
Describe the factors influencing soil formation
Information about soil formation can lead to better soil classification and more accurate interpretation of soil properties.There are several factors responsible for soil formation. The factors include climate, living organisms, relief (topography),parent material and temperature. All the factors, except time,depend to a greater or lesser extent upon each other, upon the soil itself or upon some other factor. None of the factors can be considered more important than any other, but locally one factor may exert a particular strong influence. These factors are explained in details below.
Parent material
Parent materials are made up of mineral material or organic matter or a mixture of both. The organic matter is usually composed predominantly of unconsolidated, dead and decaying plant remains. The mineral material, which is the most widespread type of parent material, contains a large number of different rock– to form Climate which decays to form results in weathering of influences the type of climate rocks vegetation humus Climate Soil mineral soil Climate forming minerals and can be in either consolidated or unconsolidated state.
Some rocks are more easily weathered than others. Acidic rocks are more resistant to weathering than basic rocks. The parent rock affects soil texture and water permeability.
Parent rock with fine particles is more resistant to chemical weathering than mechanical weathering. Very compact parent rocks like sandstone are very much resistant to weathering. Porous rocks weather easily by chemical processes. This is because they have large surface areas for weathering agents to act upon.
Climate
Climate is the principal factor governing the rate and type of soil formation as well as being the main agent determining the distribution of vegetation. The dead vegetations decay to form humus as one of the components of the soil.
To understand well the influence of climate on soil formation let us have a look at its components and how each of these components affects soil formation.
Temperature
The main effect of temperature on soil is to influence the rate of reactions; for every 10°C rise in temperature, the speed of a chemical reaction increases by a factor of 2 or 3 (twice or thrice). Temperature, therefore, influences the speed of disintegration and decomposition of the parent materials and its consolidation to form the soil.
Rainfall (water)
The water in soils includes all forms of water that enter the soil system and is derived mainly from precipitation as rain. The water entering soils contains appreciable amounts of dissolved carbodioxide, forming a weak carbonic acid. This dilute, weak acid solution is more reactive than pure water. It thus reacts with unconsolidated minerals and organic matter, breaking them down into mineral (clay, sand) and organic debris (humus) respectively.
Organisms
The organisms influencing the development of soils range from microscopic bacteria to large mammals including man. In fact, nearly every organism which lives on the surface of the earth or in the soil affects the development of soils in one way or another. More important soil organisms of interest to soil formation are as follows:
Higher plants.
Higher plants (particularly grasses) extend their roots into the soil and act as binders. So they prevent soil erosion. The roots also assist in binding together small groups of particles hence developing a crumby or granular structure. Large roots are agents of physical weathering as they open and widen cracks in rocks and stones. When plants die they contribute organic matter to the soil, which acts as a binder of the soil particles. Higher plants intercept rain and they shelter the soil from the impact of raindrops. They also shade the soil and hence reduce evaporation.
Vertebrates
Mammals such as moles, ground squirrels and mice burrow deeply into the soil and cause considerable mixing up of the soil, often by bringing up subsoil to the surface, and creating burrows through which the top soil can fall and accumulate within the subsoil.
Microogarnisms
These include bacteria, fungi, actinomycetes, algae and protozoa. These organisms act as decomposers of organic and even mineral matter.
Mesofauna
These include earthworms, nematodes, millipedes, centipedes and many insects, particularly termites and ants. Activities of mesofauna include:
- ingesting organic mineral materials e.g. earthworms and millipedes;
- transportation of materials e.g. earthworms, millipedes, termites, beetles, etc; and
- improvement of soil structure and aeration.
Man
Activities of man are too many and too diverse. Man‟s roles include:
- Cultivation of soils for production of food and tree crops, which in many cases has negative effects causing impoverishment of the soil and erosion.
- Indiscrimate grazing, casual burning, cutting of trees, manure and fertilizer use, all of which alter the soil characteristics.
Relief (Topography)
This refers to the outline of the earth‟s surface. All land surfaces are constantly changing through weathering and erosion. It may take millions of years, in the case of Himalayas and the Andes, to be worn down to flat undulating surfaces. The soils on steep mountain slopes are shallow and often stony and contain many primary minerals. In areas where the difference in elevation between the highest and the lowest point is great, then climatic changes are introduced. These differences in elevation, slope, slope direction, moisture and soil characteristics lead to the formation of a number of interesting soil sequences.
Time
Soil formation is a very slow process requiring thousands and even millions of years. Hence, it is impossible to make definite statements about the various stages in the development of soils.This is because it takes a considerable period of time for a particular soil type to be formed and categorized.
Soil Reaction
The Concept of Soil Reaction
Explain the concept of soil reaction
Soil reaction refers to how acidic or alkaline a soil is. It is expressed as a pH value. The soil can be acidic, neutral or alkaline. Extremely acidic soils can have pH values below 4.5 and on the other hand, very alkaline soils can have pH values up to,and even higher than 9.0.
Nearly all soils have pH values between 4 and 8. Soils with pH< 4 generally contain sulphuric (IV) acid, while those with pH <8 contain a high percentage of Na+ ions and thus they are alkaline.
pH is defined as the negative logarithm (to base 10) of the hydrogen ion activity (concentration):
pH = -log10[H+] where [H+] denotes the concentration of H+ ionsin grams/litre. This is the same as saying that pH is the logarithmof the reciprocal of [H+]:

The greater the [H+], the lower the pH and the more acidic the soil is. Acidic soils are common in humid regions, particularly the tropics, where rainfall is sufficiently high to leach the exchangeable bases from the top soil. Alkaline soils, on the other hand, are characteristic of the arid regions of the world where,because of low rainfall, there is a high concentration of basic cations (Ca2+, Mg2+, Na+, etc) in the surface soil layer. Generally,acidic soils occupy a large area of arable land than alkaline soils do. Because of this, acidic soils are considered to be more important, at the practical level, as compared to alkaline soils.
Causes of soil acidity
Having known that the soil can be acidic and that the acidity is a result of great concentration of H+ ions in the soil, let us know look at the causes of soil acidity. The causes of soil acidity include the following:
- Leaching: Heavy rains may leach bases like Ca2+, Mg2+, K+ andNa+ from the soil to the ground water table, leaving a surplus ofH+(aq) in the soil.
- Soil Microorganisms and root respiration produce carbon dioxide which forms weak carbonic acid with the soil solution.
- Near industrial regions, acid rain (often pH 2–4) may bring sulphuric (IV) acid and nitric (V) acid to the soil.
- Acid mineral fertilizers, like ammonium sulphate (VI) andammonium chloride make the soil solution more acidic due to oxidation and hydrolysis:
Oxidation:
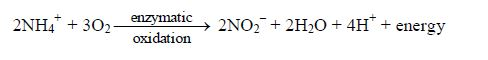
Hydrolysis:
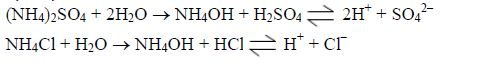
Also the NH4OH produced by oxidation and hydrolysis dissociates further to give NH4+ and OH– ions. The NH4+ ions produced undergoes microbial and enzymatic oxidation (as show above) to release more H+ ions to the soil.
5. Nitrification of ammonium ions by bacteria produces H+(aq):
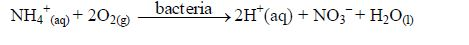
6. Organic acids produced during the decomposition of organic matter also contribute to soil acidity. Due to such reasons, most soils in the humid tropics are acidic.
7. The Al3+ ions present in soil solution contribute to soil acidity indirectly when they are hydrolysed:

For simplicity and easy understanding, the equation for hydrolysis of Al3+ ions is sometimes represented in a single equation as:
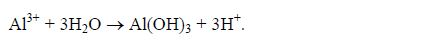
8. Small amounts of ions such as NO3‾, NO2‾, H2PO4‾, SO42‾and Cl‾ present in the soil solution also contribute to soil acidity.
The above causes to soil acidity can be categorized as either natural or artificial. The artificial causes are acid rain and acid mineral fertilizers which are a result of deliberate human actions. The rest of the causes are classified natural because they occur naturally.
The pH of a Given Soil Sample
Measure the pH of a given soil sample
The pH of a soil can be tested by using Soil pH Kit. The kitcomprises of equipment and dyes (pigments) that are employed insoil pH determination.

Soil pH kit is simple to use and can be used many times. Colour indicator dye and chart easily helps to find out pH. Knowledge of soil pH is very important to a farmer. Most plants grow well in soils with pH ranging between 5.5 and 7.5.
The following is a quick and accurate way to find soil pH using a soil pH kit:
1. Getting prepared
First collect your equipment
You will need a clean trowel and a clean container for each sample you take. You can use clean boxes, plastic bags, or any convenient container to collect your sample. For powder-based kits you will need distilled water for the test itself.
Decide where to take the samples
Soil pH can vary in different parts of your garden either naturally or through different types and levels of cultivation. You may be able to see clear differences in colour, texture and humus content. Therefore, aim to take a number of samples from different areas and test each one separately.
2. Collecting the soil samples
For each area you are sampling, scrape away the top soil to a depth of about 5 cm. This prevents the reading being affected by any top dressings or mulches you have applied or any accumulation of leaf litter or pine needles.
Now break up the soil to a depth of about 12 cm and take the sample from the bottom level. Collect more than you think you will need as you will be picking out all the lumps, stones, twigs etc. Make sure you label each sample so that you know where they have come from.

3. Sample preparation
- Pick out any stones, roots and twigs and leave the sample to dry.

- Break up the dry sample with the back of a clean teaspoon or the tip of a clean trowel, and place the specified amount in the test tube or test chamber provided.
4. Carry out the pH test
This stage will vary depending on the type of kit you have purchased.Before going any further, it is important to note that soil pH test is purely a qualitative test. In this test, barium sulphate is often used as a reagent.
Use of barium sulphate
Barium sulphate is used in soil testing as a flocculant. It causes the fine soil particles to clump together and sink, leaving a clear test solution. This enables you to make an accurate colour comparison. If you have a clay soil with lots of fine particles you may need to add extra barium sulphate to clear the liquid. This is another advantage of liquid-based tests where the barium sulphate powder is provided separately.
Liquid-based kits
If your kit contains a liquid test solution, you will usually have to add a scoop of the provided barium sulphate powder to the sample followed by the specified amount of test solution. If you have a clay-based soil it is useful to add extra barium sulphate right at the start. Put the cap on the test tube and shake it well. Leave it to settle for the required time, normally 10 minutes.
Powder-based kits
For kits containing the reagent in powder form you will have to add the specified quantity of powder (this usually contains the appropriate amount of barium sulphate as well as the reagent) followed by the required amount of distilled water. Put the cap on the test tube and shake well to mix. Allow to settle for the specified time, usually around 10 minutes.
5. Read the results
Compare the resulting colour of the solution against the supplied colour chart. Don't leave the solution for much longer than the stated time because the colour may start to change and you won't get an accurate result. Try to do this in good natural light but away from bright sunlight to make an accurate comparison.The following are the results obtained from two soil samples tested: The sample from the West Border (above) is neutral (it is neither acidic nor basic).
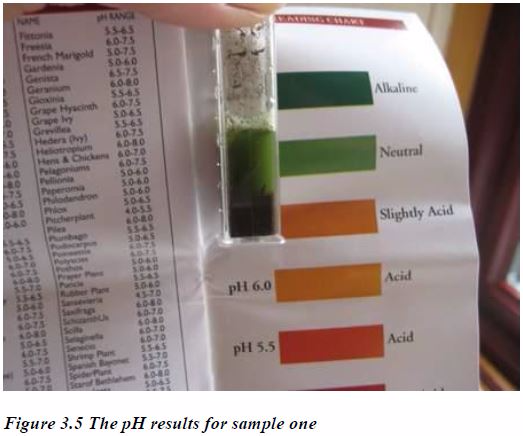
The soil sample from the North-east border, however, was found to be acidic, with pH 6.0. The soil contains a lot of organic matter, such as garden compost and rotten manure, which tends to lower soil pH, making it more acidic.
However, both soils fall well within the pH range (5.5 to 7.5) acceptable to most plants so the farmer can just carry on with farming as before. There is no need for soil pH amelioration.

Determination of the soil pH is important because it helps to identify the pH of different soils and hence making the correct decision on different kinds of crops that can be grown on a soil with a particular pH.
Plant growth is affected by the acidity or alkalinity (pH) of the soil. Soils with high peat content, or with minerals such as iron compounds, or with rotting vegetation and lack of oxygen, tend to be acidic. Their pH can reach as low as pH 4. Soils in limestone orchalky areas are alkaline (up to pH 8.3). Different plants prefer different pH conditions. Farmers and gardeners can test the soil pH to see whether it suits the needs of particular plants. An example of preferred soil pH conditions for different crops is given below:
| Crop | Preferred pH |
| Irish potato | 4.5 – 6.0 |
| Chicory, parsley | 5.0 – 6.5 |
| Carrot, sweet potato | 5.5 – 6.5 |
| Cauliflower, garlic, tomato | 5.5 – 7.5 |
| Broad bean, onion, cabbage and many others | 6.0 – 7.5 |
Managing the Soil pH by Using Different Liming Materials
Manage the soil pH by using different liming materials
If the soil is strongly acidic (pH < 5), most crops will give only very poor yields if any. In such a soil, the acidic H+(aq) and Al3+(aq) ions prevail while the basic nutrient ions such as Na+, Mg2+. Ca2+, etc are not sufficiently available.
In order to raise the pH of such a soil, basic compounds of calcium and magnesium are added and mixed well with the top soil; e.g. the oxides, hydroxides, carbonates and silicates of calcium and magnesium, commonly called agricultural limes. All these compounds have the effect of neutralizing the acidity of the soil. If the soil is too alkaline, it helps to dig
When lime is added to an acidic soil, the liming material usually reacts with the water and/or with the carbonic acid in the soil and dissolves e.g:
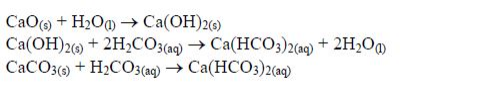
All liming materials, directly or after dissolution, react with hydrogen ions (H+) adsorbed on the soil colloids e.g:
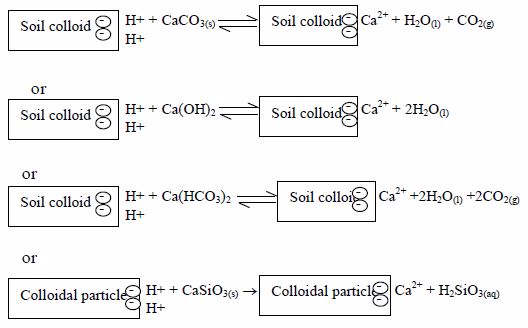
Thus, by liming an acid soil, the percentage base saturation and the pH of the soil is raised and carbon dioxide is produced. The amount of lime needed depends on the pH of the soil, its texture, structure and its content of organic matter.
Overliming, that is, raising the soil pH to above 7.5, is a danger especially in soils of low cation exchange capacity and hence low reserve acidity. It reduces the availability of P, K, Fe, Mn, B and Zn often so much that crops suffer from the deficiencies of these nutrients. Often, crops become stunted and turn yellow. Therefore, overliming must be avoided by all means.
Plant Nutrients in The Soil
The Essential Plant Nutrients
Categorize the essential plant nutrients
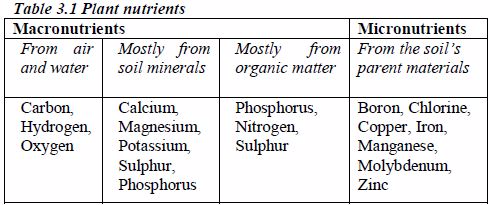
MACRO AND MICRONUTRIENTS
Macronutrients are those mineral nutrients that are required by plants in greater amounts. They constitute about 99% of plants‟ requirements.
Macronutrients, also referred to as major nutrients are further divided into primary and secondary macronutrients. Primary macronutrients are the elements that are required by plants in relatively large quantities (60% of the plant‟s requirements). They are nitrogen, phosphorus and potassium. The secondary macronutrients are calcium, magnesium and sulphur. These elements contribute the remaining 39% of the plant‟s needs
Micronutrients, also referred to as trace elements, are those mineral nutrients that are required by plants in smaller amounts. They constitute about 1% of plants‟ requirements.
Plants use the sugars for energy supply and to produce cellulose (for the cell walls) and starch (for storage). Proteins are made from sugar and nitrogen. They are used to synthesize protoplasm which is vital for the plant cells.
INTAKE OF NUTRIENTS BY PLANTS
Except for some gaseous carbon dioxide, oxygen and sulphur dioxide, all nutrients enter the plants in the form of ions usually from the soil solution through the roots.
The metals are absorbed by the plant roots in the form of their cations. Nitrogen is taken in as ammonium or nitrate (V) ions; phosphorus as dihydrogenphosphate (V) ions; sulphur as sulphate (VI) ions and chlorine as chloride ions.
The nutrient ions move towards the plant roots either with the flow of the soil solution (since the roots also take in water) or by diffusion. The ions diffuse from areas of high concentration to those of low concentration caused by the intake by the roots.
Plant roots either absorb equal numbers of positive and negative charges in the form of ions from the soil solution or they exchange one ion against another one with the same charge, usually H+ or OH–. Thus, roots possess electrical charges on their surfaces which can hold and exchange ions.
The Functions of Each of the Primary Macronutrients in Plant Growth
Explain the functions of each of the primary macronutrients in plant growth
AVAILABILITY, FUNCTION AND DEFICIENCY OF PLANT NUTRIENTS
All essential plant nutrients perform specific functions to aid plant growth or reproduction. They must all be available in the right proportion to facilitate optimum plant growth.
Carbon, Hydrogen and Oxygen
Availability: carbon and oxygen are taken in by plants through the stomata in the form of carbon dioxide from the air. Thus, they are never in scarce supply. Hydrogen (and oxygen) is supplied by water
Use in the plant: in the presence of sunlight, the green parts of the plant synthesize carbohydrates (sugars) from carbon dioxide and water.
Deficiency symptoms: plants wilt and die if they do not obtain sufficient water.
Nitrogen
Availability: only ammonium and nitrate (V) ions are available to plants. Usually about 98% of a soil‟s nitrogen is available (in organic matter). Organic matter decomposes by microbial action to form NH4+. This process is called ammonification. Almost any microbe can carry out ammonification.
There is another process called nitrification, which is carried out only by a few specific bacteria e.g. nitrosomanas. Nitrosomanas bacteria oxidize ammonium ions to nitrate (V) ions:
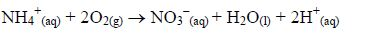
A few living organisms can fix nitrogen from the air. The best known are rhizobia (legume bacteria) and the free living bacteria such as azotobacter and clostridium, also the blue-green algae.
Plant needs: plants need more nitrogen than any other nutrient. However, their needs vary greatly: plants with high vegetative growth (stems, leaves, etc) have high N needs (maize, sorghum, rice, sugar cane, pasture grasses, most vegetables). Root crops (cassava, sweet potatoes, taro) have lower N needs. Legumes (alfalfa, desmodium, kudzu, all types of beans and peas, ground nuts, etc) may not need N fertilization if the proper strain of rhizobia is fixing N for them.
Use in the plant
- It is used to build amino acids, nucleic acids, many enzymes, chlorophyll, generally speaking: all proteins.
- It promotes the vegetative growth in plants. It is therefore important in the growth of plants in which leaves are harvested, such tobacco and vegetables.
- It is an essential element in cell division. It is therefore needed for plant growth.
- It increases grain size and protein content in cereals.
- It promotes root growth.
Deficiency symptoms: leaves turn yellow and finally die, because chlorophyll can not be build up. Then due to lack of chlorophyll the plant grows slowly, because the chlorophyll is needed for carbohydrate production. The shortage of chlorophyll is called chlorosis.
Excess nitrogen: excess nitrogen causes dark green succulent vegetation with weak stems, often at the expense of seed or fruit production, e.g. in grain crops, in tomatoes and beans.
It causes the potatoes to be watery. It delays crop maturity, and makes plants more vulnerable to attack by diseases and pests. Thus, fertilizers must be carefully dosed.
Available N is easily leached since NH4+ is rapidly nitrified in a warm climate, and NO3– is not adsorbed by soil colloids. Thus, it is important to apply fertilizer at the right time in order to avoid leaching.
Phosphorus
Availability: only H2PO4– and to a lesser extent HPO42– are available to plants.
Plant needs: plants need less phosphorus than nitrogen or potassium.
Use in plant
- Phosphorus is an essential component of the genetic material of the cell nucleus (RNA, DNA); also in ADP and ATP, which play a vital role in photosynthesis, amino acid and fat metabolism, etc.
- It increases the grain yield e.g. of millet, sorghum and rice because it promotes the formation of tillers.
- It promotes root growth.
- It strengthens the resistance of plants to diseases.
- Also rhizobia bacteria need it in order to fix nitrogen from the air.
- It hastens plant maturity.
Deficiency symptoms
- Dark green colouration
- Purple spots or streaks.
- Stunting, delayed maturity.
Fertilizers: superphosphates, triple phosphates, ammonium phosphates.
Potassium
Availability: only the K+ ions of the soil solution are available to plants. Hydrated potassium ions attached to soil colloids are readily available because they are not bonded strongly to the surface of the colloids.
After nitrogen, potassium is the second-most element needed by plants. Starch and sugar crops (cassava, sweet potatoes, banana, sugarcane) have relatively high needs of K.
Use in plants. Potassium is present in plants in the form of it ions only. It does not form any integral part of the structure of any known organic compounds in plants.
- Potassium is an activator of a number of enzymes involved amino acid synthesis and several enzymes concerned with carbohydrate and nucleic acid metabolism.
- Potassium aids in the uptake of other nutrients and in their movements within the plant e.g. potassium ions and nitrate (V) ions may move together.
- Potassium is also important in the metabolism of carbohydrates and translocation of food. Thus, it promotes starch and sugar formation.
- It regulates osmosis in cells, improves tissue formation and assists in protein synthesis.
- It strengthens plant stalk, hence preventing lodging and microbial attack.
Deficiency symptoms.
- Stunting: First the edges of the older leaves and then areas between veins turn yellow and finally brown. Small, brown necrotic spots develop while the veins are still green.
- Leaf curling and premature leaf fall.
Fertilizers: potassium chloride, potassium sulphate, potassium nitrate. Wood ashes and their aqueous extract (potash) contain potassium carbonate. Tobacco stems contain about 5% potassium, and cocoa shell meal about 3%.
Calcium
Availability: only Ca2+ ions in the soil solution are available to plants. Calcium comes from CaCO3 (calcite), gypsum (CaSO4·2H2O), apatite and other minerals.
Use in plants.
- Calcium is a constituent of cell walls and hence makes the straw stiff and resistant to lodging.
- It is essential for cell division.
- It promotes early root and seed development.
- It regulates the intake of potassium by plants.
- It neutralizes harmful organic acids like ethanedioic (oxalic) acid in plants, thus detoxifying them:
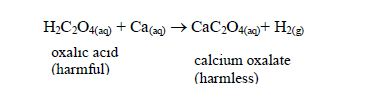
Magnesium
Availability: plants take in Mg2+(aq) through roots and leaves.
Use in plant;
- Magnesium is vital to the production of chlorophyll, because every molecule of chlorophyll contains a magnesium ion at the core of its complex structure. Most of the magnesium in plants is found in either chlorophyll or seeds. A lesser part is distributed in other parts.
- Aids in the translocation of carbohydrates.
- Regulates the uptake of other nutrients.
- Part of the distributed magnesium functions in the enzyme system involved in carbohydrate metabolism.
Fertilizers: Mostly dolomitic limestone (CaCO3·MgCO3). The principal magnesium fertilizer is magnesium sulphate (VI), sometimes known as epsom salt. It is soluble in water and can be sprayed onto the leaves.
Sulphur
Availability: Plants take in sulphate (VI) ions (SO42–) from the soil solution and sulphur dioxide from the air.
Its Use in plants.
- Sulphur is a vital part of plant proteins since cystine and methionine are sulphur-containing amino acids.
- Sulphur is also essential for the action of enzymes involved in nitrate (V) production.
The Deficiency symptoms,
Sulphur deficiency symptoms resemble nitrogen deficiency symptoms because both are related to protein and chlorophyll deficiency.
Fertilizers: gypsum and elemental sulphur, both of which are also used to lower soil pH; ammonium sulphate, superphosphate potassium sulphate (VI).
Iron
Occurrence: In igneous rocks, iron occurs in the Fe2+ form. The iron in water-logged soils tends to remain in this form and contributes to the bluish-grey colours that indicate wetness. Much of the iron in well drained soils is in the Fe3+ form and is associated with humus and mineral particles.
Availability: Plants absorb iron in the form of Fe2+ and Fe3+.
Use in plants;
Iron is an essential catalyst in the formation of chlorophyll and functions in some of the enzymes of the respiratory system. Iron is needed in larger quantity than all other micronutrients.
Its Deficiency symptoms,
An iron deficiency results in the young leaves being small and pale green or yellow in colour.
Fertilizers: Iron (II) sulphate (FeSO4) which is soluble in water. Application of iron is generally ineffective to calcareous soils.
THE OTHER MICRONUTRIENTS
Availability: Micronutrients are taken in by plants as Bo2–, Co2+, Cu2+, Mn2+, MoO2– and Zn2+. The micronutrients in the soil usually originate from the parent material of the soil. Plant needs of these micronutrients are very small.
Use in plants: Most micronutrients are used as catalysts in plant metabolism:
- Manganese is a catalyst in the formation of chlorophyll and in many redox reactions, e.g. metabolism of nitrogen, iron, copper, zinc and in vitamin C synthesis.
- Boron aids protein synthesis, regulates the K:Ca ratio in plant tissues and is required for the formation of roots and fruits.
- Copper is involved in respiration and in the nitrogen and iron metabolism.
- Molybdenum is essential in the protein synthesis and for the nitrogen fixation by rhizobia on the roots of legumes.
- Zinc catalyses the formation of growth hormones and promotes the synthesis of RNA and chloroplasts. Thus it is essential for normal growth.
- Chlorine seems to be essential in photosynthesis and is required for plant growth.
- Cobalt is essential for nitrogen fixation by rhizobia and hence aids growth of legumes. However, it is not clear whether it is essential for growth of higher plants.
Fertilization: Large amounts of micronutrients are usually toxic to plants. The best method of application is usually foliar spraying.
Preparation of Plant Nutrient Cultures in the Laboratory
Prepare plant nutrient cultures in the laboratory
Nutrient cultures are prepared in the laboratory by using chemicals such as CaSO4, Ca3(PO4)2, MgSO4 and KNO3. These salts are dissolved in water to make cultures containing ions of plant mineral elements. Different cultures lacking some mineral nutrients are made and used to grow plants. The health of plants in various cultures is compared with those grown in a culture with all elements.
Mangaging the Loss of Plant Nutrients from the Soil
Manage the loss of plant nutrients from the soil
Crop plants take up nutrients from the soil continuously. To maintain the soil fertility, the nutrients taken by plants must be replenished (replaced). There are several methods that when combined at least in some aspects can help raise or maintain soil fertility. These are:
Addition of inorganic fertilizers and manure
Inorganic (industrial) fertilizers
Fertilizers are mostly inorganic compounds which contain one or more plant nutrients in a concentrated form. They help to increase or maintain fertility if used carefully with a good background of knowledge. However, if used without proper knowledge or advice by agricultural officers they can be harmful to the soil, crops, animals and humans.
It should also be noted that without reasonable humus content, the soil may have such a low cation exchange capacity that most of the applied fertilizer is leached from the soil instead of being available to plants.
Thus, just adding fertilizer on a field without good cropping system and, advisably, with addition of manure is often a waste of money, time and energy.
Organic fertilizers (manures)
There are different kinds of manures that can be applied to the soil. These include:
- biogas manure - from biogas plants-
- farm yard manure - from wastes of farm animals such as cattle, sheep, goats, poultry, pigs, donkeys, etc;-
- compost manure – from decomposed organic matter; and-
- leguminous green manures, like sunhemp, beans, cowpeas, groundnuts, peas, etc. These young plant materials when ploughed and incorporated into the soil provide organic matter and nitrogen.
Prevention of soil erosion
Most plant nutrients are concentrated on the top soil. If this soil is eroded the nutrients are lost too. This can be stopped by taking soil conservation measures which include mulching terracing/ridging, deep tillage, contour ploughing, strip cropping, planting shelter belts or windbreaks, reforestation, avoiding overgrazing and overstocking, etc.
Crop rotation
This refers to the practice of planting different crops in a field in successive growing seasons. A good crop rotation is that which include leguminous crops (which fix nitrogen in the soil) followed by non-leguminous crops and vice-versa.
Intercropping
Intercropping refers to the act of planting two different crops (preferably legumes with non-legumes) on the same field. Legumes provide nitrogen to non-legumes and the non-legumes help to cover the soil to prevent erosion.
Agroforestry
This refers to mixed cropping of e.g. cereals and usually leguminous trees like Leucaena leucocephala, which provide nitrogen to the field. The trees take up nutrients from the deeper layers of the soil while the cereals take up their nutrients from the top layers. Leguminous trees provide the cereals with the humus when their leaves fall and rot on the soil. They also provide forage for animals, and firewood. Agroforestry is also one of the protections against soil erosion.
Good harvesting practices
Crop remnants harvest should not be burnt down but they must be ploughed and incorporated into the soil. This will help to maintain adequate levels of organic matter in the soil and hence reduce soil erosion. Also by burning crop residues, valuable plant nutrients (N, P and S) are lost and the chance to increase humus level of the soil is reduced.
Prevention of leaching
Leaching refers to loss of plant nutrients from the top to the bottom soil layers, following heavy rains or overirrigation. This can partly be stopped or reduced by maintaining adequate levels of soil organic matter to trap the nutrients and also by avoiding too much irrigation. It can also be stopped by avoiding overcultivation, a fact which makes the soil too loose that the nutrients are easily percolated with the soil solution to the bottom soil layers making these nutrients unavailable to plants.
Following /bush fallow
This refers to leaving the land idle to rest, a fact which allows the land time to regain its lost fertility. However this practice is only possible for farmers with plenty of land. It was an equally good practice in the past when human population was low as compared to vastness of the land at that time. It is not widely practiced today except in areas with low population density and abundant arable land.
Soil conservation
Taking soil conservation measures such as terracing, contour ploughing, mulching, deep tillage, etc. will help maintain soil fertility.
Cultivation of the appropriate crops for the soil
Different crops are suited to different soil types. If you plant crops in a wrong soil you are likely to weaken that soil and destroy its fertility status. But if appropriate crops are planted in the right soil type, chances of maintaining or sustaining the fertility of that soil is also very high.
Good farming practices
Adoption of appropriate soil amendment practice such as liming, acidification, conversion, etc helps to maintain soil fertility. For example, liming is good as it corrects soil acidity but if too much lime (overliming) is applied it results to another problem of setting on soil alkalinity. Likewise, an acid soil, no matter how much beneficial nutrients it contains, is of no use unless its acidity is corrected
Avoidance and/or control of soil pollution
Avoid dependence and overuse of agrochemicals such as pesticides, herbicides and inorganic fertilizers unnecessarily. These chemicals must be used with care and only where agricultural production is impossible without their application. This is because they contribute a great deal to soil pollution and toxification of beneficial soil organisms.
Nutrient balance maintenance
The balance of nutrients in the soil must always be maintained. Plants usually require specific quantities of different nutrient elements. These nutrients must be maintained in the soil by good cropping systems, application of appropriate fertilizers and manure and adopting good soil management practices.
Manures and Fertilizers
Fertilizer refers to any natural/manufactured/synthetic materials that contain at least 5% or more of one or more of the primary plant nutrients/element (N, P or K). Examples of fertilizers include ammonium nitrate, sulphate of ammonia, CAN, NPK, etc.
Manure refers to any fertilizer material, from plant or animal bodies or wastes. Examples of manures include farm yard manure (from domesticated animal wastes), green manure (from young green leguminous plants), and heap and compost manure (from decomposed plants). Manures are sometimes called organic fertilizers.
Preparation of Heap and Pit Compost Manure
Prepare heap and pit compost manure
Steps:
- Dig compost pit;
- Place dry plant materials. Sprinkle enough water;
- The next layer will be composed of green plant materials or any refuse
- Top this with a mixture of animal manure, soil, and ash;
- Repeat steps 2-4 until the pile reaches a height of 1 m;
- Cover the pit with broad-leaved plants;
- Turn the pile every two weeks. The compost is ready after 3-4 months.
The Advantages and Disadvantages of Natural Manures
Explain the advantages and disadvantages of natural manures
Manures have got several advantages and disadvantages. Some of these are explained below:
Advantages
- They add nutrients to the soil and at the same time improve soil physical properties such as soil colour, soil structure and water holding capacity of the soil. A soil with good content of organic matter (supplied by manure) holds water and dissolved nutrients efficiently making them available to crop plants.
- Manures supply humus to soil which, in turn, increases the cation exchange capacity of the soil. Humus accounts for 30–90% of the cation exchange capacity of mineral soils. And because of its high cation exchange capacity, humus helps to store nutrient cations, especially ammonium ions, thus reducing the leaching of these nutrients from the soil.
- Manures improve the proliferation of the soil macro- and microorganisms by supplying the nutrients and conducive conditions needed by these organisms for survival. These organisms play a vital role in soil fertility and plant nutrition by decomposing organic matter which releases nutrients to the
- Manures provide organic matter which acts as the binding materials for soil particles, making them more compact and hence resistant to the impact of rain drops and surface run off of water. Thus, it reduces soil erosion.
- Manures can remain in the soil for a long time and they can provide the nutrients to crops for several growing seasons. Its nutrients are released slowly over a long period of time
- They do not change the soil pH greatly as the inorganic fertilizers do.
- Humus from organic matter is dark in colour and imparts this black colouration to the soil. Black colour absorbs more heat and hence helps to regulate soil temperature.
Disadvantages
- Manures contain and provide little nutrients per unit volume and weight. One has to apply tremendous amounts of manure to meet the requirements of plants.
- Their bulkiness and volume makes it difficult to store, handle or apply in the field. It requires more space to store or transport and more labour to apply manures in the field.
- Some kinds of manures e.g. sludge or industrial and municipal wastes may contain toxic chemicals which can harm humans, animals or soils to which it is applied.
- Manures act very slowly in that they release nutrients to the soil at a very slow rate.
- If the plant materials used to make manure is infested with plant pests or weed seeds; or infected with diseases, there is a risk of spreading them to the farm.
- Manures easily lose nutrients if stored improperly. Under hot conditions, manures produce a lot of heat that leads to loss of136nitrogen through vapourization. Soluble nutrients are easily leached
Types of Synthetic Fertilisers Used in Tanzania
Mention types of synthetic fertilisers used in Tanzania
Farmers in different parts of the country use different fertilizers (depending on soil conditions) to improve soil fertility. Examples of fertilizers used by farmers in Tanzania include:
- Sulphate of ammonia, (NH4)SO4;
- Calcium Ammonium Nitrate, CAN;
- NPK;
- Superphosphates;
- Ammonium chloride, NH4Cl;
- Urea, CO(NH2)2
- Ammonium nitrate, NH4NO3;
- Potassium sulphate, K2SO4
- Potassium chloride, KCl; etc.
The Concept of Fertiliser Grades and Analysis
Explain the concept of fertiliser grades and analysis
A fertilizer is any substance containing plant nutrients that is usually added to soil to supplement the required plant nutrients. Chemical fertilizers may be natural or synthetic. Natural inorganic fertilizers would include materials such as Chile saltpetre (NaNO3), rock phosphate, potassium chloride (KCl), etc. Synthetic fertilizers are manufactured products, such as urea, ammonium sulphate, ammonium phosphate, single superphospahte, etc.
Synthetic fertilizers are available in various grades and analyses. A complete fertilizer has all three primary fertilizer ingredients (N, P and K) as part of its formulation. For example, 14 – 15 – 14 is a complete fertilizer. This fertilizer has 14%N, 15%P and 14%K.
Fertilizers may be classified according to the nutrient elements present. Fertilizers may be:
- Single nutrient fertilizer or straight fertilizers: These are fertilizers containing only one of the primary nutrient elements.
- Double nutrient fertilizer: These contain two primary nutrient elements.
- Complete fertilizers or complex fertilizers: These are materials that contain all three primary elements, N, P and K.
Types of fertilizers
Now let us look at few common fertilizer materials and their grades.
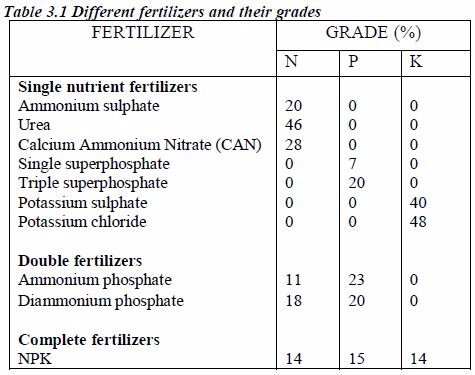
In most of the commercials fertilizers P is expressed as P2O5 and K as K2O. To convert P2O5 to P, multiply the value by 0.437 and to convert K2O to K, multiply the value by 0.830. However, in table 3.1 the nutrient contents are given in elemental form, N, P and K.
The nutrient fertilizer grade indicates that everything in a bag of fertilizer is not plant nutrient. Much of the material in the bag is made up of filler materials. For example, ammonium sulphate contains 20%N. That means, 100 Kg of ammonium sulphate will contain 20 Kg of N and the remaining 80 Kg is sulphate and filler material.

With this background about fertilizer material, we need to calculate the amounts of each nutrient source to meet the fertilizer recommendations. Fertilizer recommendations are expressed in kilograms of nitrogen (N), phosphorus (P) and potassium (K) per hectare. Fertilizers available in the market also contain carrier and filler materials in addition to N, P, or K. Hence, there is need to compute the amount of fertilizer required to supply the recommended rate of nutrients.For example, if 90 Kg of N is recommended for sorghum, we must convert this recommendation to Kg of urea or some other N fertilizer to be applied.
Example 1
A certain soil requires 80 Kg of N per hectare so as to fulfil plantrequirements of nitrogen. Calculate, in kilograms, the quantity ofammonium sulphate fertilizer required to meet this demand.
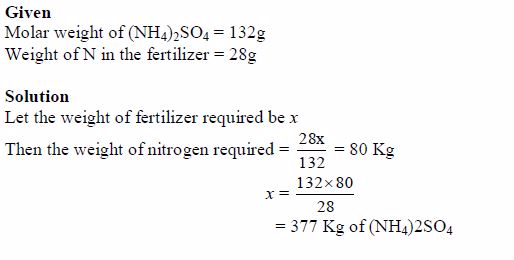
Methods of Fertilizer Application
Identify methods of fertilizer application
Fertilizers can be applied in several ways. The most important point to remember is to apply them at the proper rate, as over application can result in plant damage or death. Follow soil recommendations or manufacture‟s directions. Some of the common fertilizer application methods are as follows:
Broadcasting
Broadcasting refers to spreading the fertilizer uniformly over theentire area before planting and then incorporating it into the soil.Application of high rates of the major nutrients namely, nitrogen,potassium and phosphorus is usually carried out by broadcastingwith a tractor-mounted fertilizer spreader. This allows a specific amount of fertilizer to be spread over the entire under-tree area or in band along the row.
Monitoring of the application rate for the various fertilizers which are used is important. If calibration is not done properly, the quantity applied may be considerably different to the desired rate.
Soon after spreading, the fertilizer should be covered with the surface soil to a depth of 4 to 6 inches or alternatively watered in. On alkaline soils, prompt incorporation into the soil profile is vital for nitrogenous fertilizers as they are readily lost into the atmosphere (volatized) in the form of ammonia if left exposed on the soil surface.
Broadcasting is particularly good:
- In soils whose fertility status is extremely low;
- In closely spaced crops such as rice, wheat, pasture, millet, etc;
- If the fertilizer used is in fine particles (granules or powdered); and
- If the field is properly prepared and is in good tilth.
Fertigation
Fertigation refers to the application of nutrients through an irrigation system. In this method, liquid fertilizers such as liquid ammonia, nitrogen solutions, phosphoric acids and even complete fertilizers are applied to the soil via irrigation water. The nutrients are spread onto the soil in solution and then carried down with the infiltrating water. It provides a simple and effective way to supply nutrients, particularly nitrogen and potassium. Most trickle, jet, spray and sprinkler systems can be used. Do not attempt fertigation using flood or furrow irrigation as the distribution of nutrients will be uneven and leaching will occur.
Fertigation has the advantage of supplying nutrients to the area of greatest root activity that is, the irrigated part of the root zone.Depending on the irrigation system used, applications of fertilizer may need to be split over several irrigations. This also improves the percentage taken up and utilized by the plant. Frequent applications are easy to apply and there is no soil compaction problem as would be the case with broadcasting with a tractor.
Before injecting a nutrient into an irrigation system ensure that the form being used is suitable. Some nutrients are readily soluble in irrigation water while other nutrients must be specially formulated for fertigation. For example, most forms of phosphorus are not suited to fertigation as they tend to precipitate out and block the small orifices of irrigation emitters.
Foliar sprays/applications
This refers to spraying a dilute nutrient solution directly to the plant leaves. Foliar application is used for the correction of trace element deficiencies. It should not be relied upon to supply the total nitrogen, phosphorus, and potassium needs of plants. Commonly required foliar sprays are zinc and manganese. Foliar application has the benefit of rapid response as the nutrients are taken into the plant quickly. Foliar applications of micronutrients, especially iron, may be beneficial when high soil pH conditions make the iron unavailable to plant roots.
Generally, uptake is enhanced by low pH, thus there is benefit in adjusting the pH of the spray solution to slightly acidic.
Often foliar applications are more effective in correcting trace element deficiencies than soil applications. Trace elements are frequently tied up by unfavourable soil condition so that nutrients applied to the soil are bound before the plant can utilize them while foliar application bypasses this problem.Foliar spray can also be used to alleviate deficiency of major elements. This is will provide only temporary relief and should be used only as a quick fix to minimize yield or quality loss due to a sudden deficiency. It is not possible to apply the quantity of the major nutrients that growing plants require by foliar applications. Soil applications should provide over 90% of the quantity of each major nutrient.
Banding
Banding refers to placement of fertilizer 2 to 3 inches to each side and below the seed at planting. This technique is risky as placement too close to the seed or at high rate can cause fertilizer burn and inhibit germination.
This method is ideal:
- where the amount of fertilizer to apply is limited;
- for widely-spaced crops;
- when small labour is required ; and
- in seedbed preparation.
Side-dressing
Side-dressing refers to placing the fertilizer beside the row during the growing season. This technique is usually used to apply additional nitrogen during the growing season and is particularly useful for applying nitrogen on sandy soils.
Top-dressing
Top-dressing is similar to side-dressing except that the fertilizer is applied around the plant. Care must be taken not to apply the fertilizer too close to the plant as this can cause fertilizer burn.
Starter Solution Application
Starter solution fertilizers are soluble in water and they are usually high in phosphorus. They are applied as liquid around the plant roots at the time of planting. They are primarily used for vegetable transplants to hasten root development and establishment. Follow manufacturer‟s directions for application rates.
The Advantages and Disadvantages of Artificial Fertilizers as Compared to Natural Manures
Explain the advantages and disadvantages of artificial fertilizers as compared to natural manures
Advantages
- They contain more nutrients per unit volume and weight.
- They are compact and hence easy to transport, store and apply to the field as compared to manures which are bulky in nature.
- They contain specific quantities of plant nutrients per unit volume and weight. So the quantity of a particular nutrient to be applied to the soil can easily be estimated to avoid overapplication. For example the weight of nitrogen in a kilogram of NH4NO3 can easily be established and quantified.
- They dissolve quickly and hence provide nutrients to plants instantly as they are added to the soil.
Disadvantages
- They are used only for one growing season as they are short-lived. Because the uptake of the nutrients in the fertilizer is very high, no or few nutrients would have remained in the soil in the next growing season.
- Some acidic mineral fertilizers such as NH4Cl and (NH4)2SO4 contribute to soil acidity. When these fertilizers are applied to the soil repeatedly, they can make the soil acidic and hence not fit for plant growth.
- Prolonged use of artificial fertilisers may lead to deterioration of the soil structure and poisoning of soil and soil microbes.
- Extensive use of fertilizers may cause contamination of drinking water resources, by especially nitrate fertilizers. Nitrates dissolved in water are not removed by normal purification processes. In the body nitrates may be converted into nitrosamines. These are carcinogenic (cancer-causing) compounds
- If too much nitrogenous fertilizer is used, serious pollution can occur. One kind of pollution is called eutrophication. Excess of the fertilizer applied finally finds its way to water. This encourages fast growth and huge increase in the number of microscopic, aquatic plants called algae, a phenomenon called algal bloom. Proliferation of algae on the water surface blocks sunlight from reaching the plants beneath the water. These plants can not carry out photosynthesis and, therefore, they die. Bacteria and other decomposers feed on these dead plants and increase in number. These decomposers use up all the oxygen dissolved in the water. Without ample supply of oxygen fish and other organisms living in the water die.
- They can scorch (burn) and kill crop plants if not applied under manufacture‟s directions or even harm humans if not properly handled.
- Fertilizers are expensive to purchase and hence not affordable.
Soil Fertility and Productivity
The Concept of Soil Fertility and Soil Productivity
Explain the concept of soil fertility and soil productivity
Soil fertility
Soil fertility is the ability of the soil to supply the essential nutrient elements in adequate amounts, forms, and proportions for maximum plant growth.
There are three types of soil fertility. These are:
- Chemical soil fertility: this is the fertility due to chemical processes that contribute to soil fertility. The chemical soil fertility falls under two categories namely,(i) potential chemical fertility, due to cations in soil solution; and(ii) active chemical fertility, which is due to exchangeable cations adsorbed to the soil colloidal surface or negatively charged plant roots.
- Physical soil fertility: the fertility contributed by soil moisture, texture, structure, temperature, etc.
- Biological soil fertility: this is due to organic matter content, and soil microorganisms.
Soil productivity
Soil productivity is the capacity or ability of a particular soil to sustain plant growth and development. It is measured in terms of yield of a particular crop which is a reflection or consequence of nutrients taken up by plants from the soil. Soil productivity is an interaction of three main factors.
- Soil fertility. This refers to the ability of the soil to supply the essential plant nutrients required for maximum plant growth.
- Plant factors. These includes yield potential, root growth characteristic and genetic make up of a particular crop plant. This means that some crop plants are high-yielding than other plants of the same species and are thus likely to give more crop yields. Also plants with good root development are likely to absorb more nutrients from the soil, grow better and give good yield as compared to plants with poor root development. Genetic make up of a plant also plays a vital role in this respect. For example hybrid maize will always survive harsh146soil and environment conditions than local varieties of maize and, therefore, will give high yields.
- Environment factors. These factors include climatic factors and agronomic practices.Climatic factors – These are factors such as temperature, precipitation (rainfall), radiation, humidity, altitude, etc.Agronomic practices include weed control, pest and disease control, good soil preparation, plant population, etc. Yield can be measured in terms of grain yield, tubers yield, dry matter, height of plants, number of leaves, number and size of fruits, berries, etc.
A soil is considered to productive if:
- It has adequate water retention capacity;
- It is well aerated; and
- It is able to supply adequate amounts of the nutrients to plant.
Water retention capacity is influenced by soil organic matter, soil texture (loam, clay, sand, etc), soil structure, and proportions of macro and micro pores in the soil. Aeration is influenced by soil structure and texture.
Difference between Soil Fertility and Soil Productivity
Differentiate soil fertility form soil productivity
We have learned that soil productivity depends on soil fertility in one way or another. However, plant and climatic factors have their roles play too.
From this point of view, therefore, it is correct to assert that a fertile soil is not necessarily productive simply because soil productivity does not rely singly on the fertility of the soil. It depends on several other factors such as soil moisture, which is determined by climate and even altitude, which influences plant development a great deal. In concise, it should be understood that there are several other factors apart from soil fertility which affects the productivity of the soil. Even soil management practices can affect soil productivity to a large extent. Soil fertility is accounted for by the type and quantity of the nutrient elements present in a particular soil which are available to crop plants.
Soil productivity is a measure of the amount of harvest or yield that can be obtained from a given piece of land under certain agronomic conditions and practices. For example, suppose a farmer grows maize on one acre of plot A and manages to harvest 20 bags of maize. On another one-acre plot, plot B, he harvests only 10 bags of maize. Of the two plots, A is said to be more productive than B. This is one among many means for determining soil productivity.
The Factors which Determine Fertility and Productivity of the Soil
Explain the factors which determine fertility and productivity of the soil
A fertile soil provides all essential plant nutrients in amounts and proportions which are suitable for growth of most plants. Soil fertility depends on a number of factors, namely:
The texture and structure of the soil
This affects water and nutrient storage, and aeration. The soil with a fine texture such as clay contains small airspaces. The movement of air in and out of such a soil is thus minimal. However, these soils have a great capability of holding water and nutrients. Its structure can be corrected by addition of organic matter, such as farm yard manure and compost, and heap manure.
On the other hand, soils having a coarse texture such as sand are quite porous. Sand allows water to pass through it very quickly. It is poor at water retention and nutrient storage. It has wide air spaces and thus well aerated. It can also be improved by adding organic manures.
The depth of the soil profile
The deeper the soil the better the plant root development and the greater the water and nutrient supply potential it has. Shallow soils do not normally allow roots to penetrate deep through the soil. This leads to poor root development and poor plant growth.
The mineral and organic matter content
The chemical composition of the parent material of the soil provides the natural inorganic nutrient supply due to minerals present. A soil formed from the decomposition of limestone will probably contain reasonably high concentrations of Ca2+ ions in their exchangeable sites due to inherent Ca2+ ions derived from limestone. The same case can apply to high contents of nitrogen and phosphorus in humid soils due to high decomposition of organic matter. The soil organic matter helps to cement the soil particles therefore aiding to create a crumbly structure which is ideal for most agronomic practices.
Cation Exchange Capacity (CEC)
This depends very much on the content of the soil and soil pH. A soil well supplied with organic matter has an optimum cation exchange capacity. The cations adsorbed to the soil colloids are easily exchanged with those in the soil solution. Also humus contains humic (organic) acids which can donate protons if the pH is low.
Soil pH
This affects nutrient storage and availability. The availability of N, P, K, S, Ca, Mg, and Mo decreases with increase in soil acidity. Below pH 5 and above pH 7, Al3+ and Fe3+ ions form complexes with soluble phosphates so that the phosphates are no longer available to plants. This is called phosphorus fixation. Below pH 4.8, Al3+ becomes so soluble that it appears in high concentrations in the soil solution which are detrimental to most plants. This aluminium toxicity is a problem in some tropical soils.
Climate
The climate affects water availability, temperature, weathering as well as the physical and chemical properties of the soil. In humid tropical climates the rate of weathering and organic matter decomposition is very high. Soils in wet tropical and equatorial regions are well supplied with water because these regions normally receive sufficient rainfall.
The position of the ground water table
Water table position affects drainage. Normally, the lower the water table the wet is the soil. Such soils have a good content of moisture and soil microorganisms. Usually water rises from the bottom to the upper parts of the soil profile by capillarity action. The presence of a water table can be detected by vegetations growing directly above it, which always remain greenish even during the dry spell.
The Causes of Loss in Soil Fertility
Explain the causes of loss in soil fertility
All factors that contribute to loss of nutrients from the soil cause loss in soil fertility. These factors include:
- Soil erosion: Most plant nutrients are contained in top and sub soil. It is these nutrients that are available to plants for growth and development. When the top player of the soil is removed by erosion, the nutrients in it are lost too. Erosion agents tend to clear and transport the soil from its original site to another site far away. By so doing, the nutrient elements that are contained in this soil are also carried together with the soil. This process then leads to loss of nutrients from the soil and hence loss in soil fertility.
- Leaching: This refers to the flushing of plant nutrients from the top to the lower layers of the soil and beyond the reach of plant150roots. This is caused by heavy rainfall or flood irrigation. The process makes the nutrients unavailable to plants as it washes them for beyond the root zone.
- Monoculture: The cultivation of the same type of crop on a piece of land year after year, leads to soil depletion if manures or fertilizers are not added. Different plants have specific needs for particular mineral compounds. If the same type of crop is grown on as similar field continuously over a number of years, then the soil will become deficient of the minerals taken up by that crop.
- Denitrification: Denitrifying bacteria convert nitrates of the soil to gaseous nitrogen which escapes to the atmosphere, thus depriving the soil of nitrogen. However, this process is counteracted by another type of beneficial bacteria called nitrifying bacteria, which again convert nitrogen of the air back to soil nitrates by a process called nitrification.
- Nutrient uptake by plants: Growing plants absorb nutrients from the soil for growth and development. If the nutrients taken up by plants are not replaced through adding manure or inorganic fertilizers, the soil becomes deficient of these minerals.
- Volatilization: This refers to the conversion of ammonium compounds into ammonia gas. Nitrogenous compounds in the soil are decomposed by heat into ammonia gas. Also when nitrogenous fertilizers are added to highly basic soils, they react with sodium hydroxide in the soil to release ammonia gas which simply escapes to the atmosphere.NH4+(aq) + OH–(aq) → NH3(g) + H2O(l)
- Accumulation of salts: Under normal conditions, rain water washes the mineral salts away, thereby keeping their concentrations in the soil low. However in arid and semi arid151regions the salts accumulate in the soil as the rain falling there is irregular and is insufficient to wash away the salts. This, together with the high evaporation rate and poor drainage, leads to excessive accumulation of salts on or below the soil surface.
- Change in soil pH: Use of acidic fertilizers over a long period of time can make the soil acidic. Change in soil pH affects the activity of soil microorganisms and availability of some plant nutrients. This, in turn, affects the fertility of the soil.
- Burning of vegetation: Burning crop residues and vegetation deprives the soil microorganisms of the organic matter they require for survival and proliferation. This affects microbial activities such as nitrogen fixation and decomposition of organic matter
The act of burning vegetation also exposes the soil to erosion agents such water and wind. The resulting ash also may cause imbalance of nutrients in the soil or make the soil alkaline.






EmoticonEmoticon