Join Our Groups
TOPIC 9: COMPOUNDS OF METALS
Metals combine with other substances to form compounds. There is a diversity of metal compounds known. One can make an endless list of the compounds of the metals. In this chapter, we are going to concentrate our efforts on the following compounds of the metals: oxides, hydroxides, carbonates and hydrogencarbonates, nitrates, chlorides and sulphates.
Oxides
Preparation of Oxides of Some Metals by Direct and Indirect Methods
Prepare oxides of some metals by direct and indirect methods
All elements except helium, neon and argon form compounds with oxygen. This is because oxygen is quite reactive. Binary compounds of oxygen are known as oxides. Therefore, a metal oxide is a binary compound of oxygen and a metal.
Metal Oxides
Classify metal oxides
Chemical properties of metal oxides
- Reaction with water: The oxides of potassium, sodium and calcium are very soluble in water. They will react vigorously with cold water to produce the corresponding hydroxides. The oxides of metals below calcium in the reactivity series are all insoluble in water.
- Reaction with acids: The oxides of metals above hydrogen in the reactivity series react with dilute acids to produce salt and water.
- Reaction with alkalis: Some oxides react with alkalis to produce salt and water. The oxides of this nature include ZnO, Al2O3, PbO and SnO.
The Reactions of Metal Oxides with Water and Dilute Acids
Demonstrate the reactions of metal oxides with water and dilute acids
Preparation of metal oxides
Metal oxides can be prepared by:
- direct methods. This involves heating metals directly in air.
- indirect methods. This involves such methods like heating carbonates and hydrogencarbonates of certain metals in air, and reacting certain metals with certain acids.
Preparation of metal oxides by direct combination(Direct method)
In this method, oxides can be prepared by direct combination of metals with oxygen. This involves heating a metal in air. When some metals are burned in the air, they react with oxygen of the air to form metal oxides. However, this method is not intensively used because some metals tend to form a protective layer of an oxide on the surface of metal and prevent further attack by oxygen. The best example of such metals is aluminium which, when heated in the air, forms a protective layer of aluminium (III) oxide (Al2O3) on the surface of a metal whichprevents further attack by oxygen. Table 9.1 shows the products formed when certain metals are burned in the air.
Table 9.1: The reaction of metals with oxygen
| Metal | How it reacts | Product |
| Barium | burns with a green flame | white solid (barium (II) oxide, BaO) |
| Calcium | burns with a brick-red flame | white solid (calcium oxide, CaO) |
| Sodium | burns with a yellow flame | white solid (sodium oxide, Na2O) |
| Potassium | burns with a purple flame | white solid (potassium oxide, K2O) |
| Magnesium | burns with a white flame | white solid (magnesium oxide, MgO) |
| Iron | burns with yellow sparks | Blue-black solid (iron (II) oxide, FeO) |
| Copper | doesnot burn, turns black | black solid (copper (II) oxide, CuO) |
Preparation of metal oxides by indirect methods
This method involves thermal decomposition of salts. When some salts are heated, they decompose into oxides and other products as well. If the anion part of the salt heated contains some oxygen, a portion of this oxygen may remain bonded to the central metal atom.
However, this method is limited only to those compounds of metals below sodium in the electrochemical series. For instance, potassium oxide or sodium oxide cannot be prepared by action of heat on their carbonates. Thermal decomposition of some metals is as shown by the following equations:
- CuCO3(s)→CuO(s)+ O2(g)
- CaCO3(s)→CaO(s)+ CO2(g)
- ZnCO3(s)→ ZnO(s)+ CO2(g)
Hydroxides also behave in a similar manner. When hydroxides of metals below sodium in the electrochemical series are heated, they decompose into respective oxides, giving off water in the form of steam.
- Ca(OH)2(s)→ CaO(s)+ H2O(g)
- Mg(OH)2(s)→ MgO(s)+ H2O(g)
The oxides can also prepared by heating some nitrates and sulphates:
- 2Pb(NO3)2(s)→ 2PbO(s)+ 4NO2(g)+ O2(g)
- 2Cu(NO3)2(s)→ 2CuO(s)+ 4NO2(g)+ O2(g)
Nitrates of silver and mercury are not suitable for preparation of oxides by thermal decomposition because they decompose to metals directly when heated. The bond between oxygen and a metal atom is not strong enough to withstand the thermal energy:
- 2AgNO3(s)→ 2Ag(s)+ 2NO2(g)+ O2(g)
The Uses of Metal Oxides
Explain the uses of metal oxides
Metal oxides find a wide range of uses. The following are the uses of the most common oxides:
Uses of calcium oxide (CaO)
- Making mortar:Calcium oxide reacts with water to form the hydroxide, Ca(OH)2, known as slaked lime.Mortar is made by mixing slaked lime, sand and water, and is used in sticking bricks together and in forming smooth surfaces on walls of buildings.
- Calcium oxide is used for making whitewash, which is used in marking sport’s fields, roads and is brushed on walls of buildings to give them the white colour. Whitewash is a suspension of slaked lime in water.
- Cement and concrete: Cement is made by heating together lime or limestone and clay. The product is a mixture of calcium silicates and aluminates. Clay is hydrated aluminium silicate. A mixture of cement, sand, stones and water gives concrete, which on setting becomes extremely hard. It is the materials used for making foundations of buildings, pillars, roads, paths, bridges, etc
- Soil treatment:In agriculture, quicklime is used to neutralize soil acidity, and it also adds mineral nutrients (Ca2+) to the soil.
- Calcium oxide is dissolved in water to make slaked lime, which is used in the softening of water.
- Drying agent: Calcium oxide is used for drying ammonia and ethanol.
- It is used in the manufacture of bleaching powder, CaOCl2.
- Manufacture of glass: heating a mixture of sand, sodium carbonate and lime or limestone gives glass.
- Lining of furnaces: It is mixed with magnesium oxide to form the basic lining of the furnaces to remove acidic impurities in the form of slag.
- it is used in the blast furnace to remove impurities fromiron ore which is removedin the formof slag.
- Preparation calcium carbide: Calcium carbide (CaC2) is manufactured in an electric furnace at 2000oC. CaO(s)+ 3C(s)→ CaC2(s)+ CO(g)
Uses of magnesium oxide (MgO)
- The oxide is used as a lining material in refractory furnaces, owing its high melting point, which is around 2900oC. It is also used as a refractory agent in the construction of crucibles.
- The oxide in its solution form (magnesium hydroxide) is commonly used as an antacid. This works because magnesium hydroxide is a basic substance, which means that, it will neutralize excess acidity and end up indigestion, caused by too much hydrochloric acid in the stomach.
- It is used to manufacture the common chemical reagents in the laboratory such a magnesium chloride, magnesium sulphate and magnesium hydroxide.
- Magnesium oxide is a popular drying agent. In its powder form, it is hydroscopic in nature. This makes it suitable for drying different substances.
- Insulation: Due to its heat resistance properties, magnesium oxide powder makes an excellent insulator.
- Dietary supplement: Since it is a good source of magnesium, the oxide is used as or in dietary supplements forhumans and animals.
Uses of aluminium (III) oxide (Al2O3)
- Aluminium (III) oxide, in the form of bauxite, is used as a source of aluminium.
- Owing its rough surface, the oxide is used as an abrasive, i.e. it is used to rub and clean other surfaces.
- It is used as an adsorbent in chromatography.
- It is used in the lining of furnaces as a refractory material because it has a high melting point (2040oC).
Uses of zinc oxide (ZnO)
- Zinc oxide is chiefly used in the manufacture of paints and pigments. In addition, the oxide is used to manufacture anti-corrosive coatings, lubricants, adhesive batteries, fire retardants, plastic, cement, glass and ceramics (as a component of glazes).
- Manufacture of rubber: It is mainly used to activate vulcanization, which aims at improving the strength and elasticity of rubber.
- Manufacture of cigarette filter: As a cigarette filter, zinc oxide helps to remove certain harmful compounds from the tobacco smoke, without altering its flavour.
- Making concrete: It helps to make the concrete more resistant to water, besides improving the processing time required.
- Medical uses: Zinc oxide has anti-bacterial properties, for which it is extensively used to treat a number of skin conditions. It is topically applied to provide relief in skin irritation, diaper rash, minor burns and cuts, and for dry and chapped skin. It is added to baby powder, anti-dandruff shampoos as well as antiseptic creams and surgical tapes due to its medicinal properties. In addition, together with iron oxide, it is used to make calamine solution.
- Cosmetic uses: the most important use of zinc oxide in the cosmetic industry is in the preparation of sunscreen lotions and creams. Zinc oxide can absorb ultraviolet (UV) radiation of the sun and thereby protect the skin from sunburn and other damaging effects of UV radiation.
Hydroxides
Preparation of Hydroxides of some Metals by Direct and Indirect Methods
Prepare hydroxides of some metals by direct and indirect methods
Metal hydroxides are electrovalent compounds, composed of metallic ions, which are positively charged, and hydroxyl ions, OH-, which are negatively charged. The nature of the hydroxides of the metals varies according to the position of the metal in the reactivity series, as shown below:

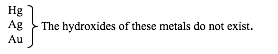
Metal hydroxides can be prepared in the laboratory by two methods:
- Direct method
- Indirect method
Direct method
This method involves such processes like adding metals directly in water. The method is suitable for preparation of soluble hydroxides (alkalis).
- 2Na(s)+ 2H2O(l)→ 2NaOH(aq)+ H2(g)
- 2K(s)+ 2H2O(l)→ 2KOH(aq)+ H2(g)
- Ca(s)+ 2H2O(l)→ Ca(OH)2(aq)+ H2(g)
Indirect method
This method involves the preparation of insoluble hydroxides by reacting aqueous solutions of sodium or potassium hydroxide with aqueous salts of metals. These are called precipitation reactions. Only NH4OH, KOH and NaOH are completely soluble in water. Calcium hydroxide is sparingly soluble in water (0.173g/100 ml at 20°C). All other hydroxides are insoluble in water and they can be prepared by this method:
- CuCl2(aq)+ 2NaOH(aq)→ Cu(OH)2(s)+ 2NaCl(aq)
- Ionically:Cu2+(aq)+ 2OH-(aq)→ Cu(OH)2(s)
- FeSO4(aq)+ 2KOH(aq)→ Fe(OH)2(s)+ K2SO4(aq)
- Ionically:Fe2+(aq)+ 2OH-(aq)→ Fe(OH)2(s)
- FeCl3(aq)+ 3NaOH(aq)→ Fe(OH)3(s)+ 3NaCl(aq)
- Ionically:Fe3+(aq)+ 3OH-(aq)→ Fe(OH)3(s)
Metal Hydroxides
Classify metal hydroxides
Just like metal oxides, metal hydroxides can be classified based on their solubility in water as either soluble or insoluble hydroxides. They can also be classified as basic or amphoteric hydroxides, based on their reaction with acids and bases.
The classification of hydroxides as either soluble or insoluble is as shown on the previous page.
Based on their reaction with acids and bases, metal hydroxides are classified into basic and amphoteric hydroxides.
A basic hydroxide is a metal hydroxide that contains hydroxyl ions, OH-, and will react with an acid to form a salt and water only. An amphoteric hydroxide is a hydroxide that shows both acidic and basic properties, that is, will react with both an acid and a base.Examples of amphoteric hydroxides are Al(OH)3, Zn(OH)2and Pb(OH)2.
Amphoteric hydroxides behave as bases and as acids under different conditions. These hydroxides are only weakly basic, but like other bases, they still combine with acids to form salt and water:
- Zn(OH)2(s)+ H2SO4(aq)→ ZnSO4(aq)+ 2H2O(l)
- Pb(OH)2(s)+ 2HNO3(aq)→ Pb(NO3)2(aq)+ 2H2O(l)
- Al(OH)3(s)+ 3HCl(aq)→ AlCl3(aq)+ 3H2O(l)
However, in the presence of strong alkalis, these hydroxides behave like acids, and they combine with these strong alkalis to yield a salt and water:
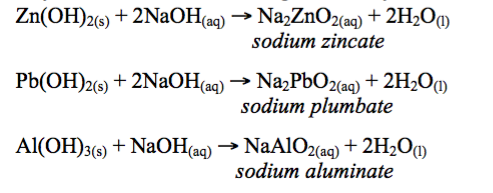
The Chemical Properties of Metal Hydroxides
Explain the chemical properties of metal hydroxides
In this particular case, the chemical properties of only a few common hydroxides will be discussed.
Chemical properties of sodium hydroxide (NaOH) and potassium hydroxide (KOH)
When heated together with aluminium, zinc and lead metals, concentrated sodium hydroxide dissolves these metals to form sodium aluminate, zincate and plumbate respectively.
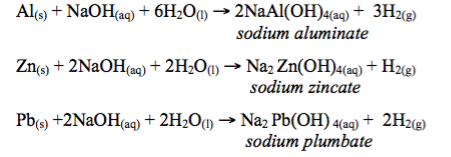
Caustic alkalis react with soluble salts of certain metals like copper, lead, zinc and iron to form their insoluble hydroxides by double decomposition.
- 3NaOH(aq)+ FeCl3(aq)→ Fe(OH)3(s)+ 3NaCl(aq)
- 2NaOH(aq)+ ZnSO4(aq)→ Zn(OH)2(s)+ Na2SO4(aq)
Sodium hydroxide liberates ammonia gas from ammonia salts when both are warmed together. This is the standard test for any ammonium salt.
- NH4Cl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)+ NH3(g)
- NH4NO3(aq)+ NaOH(aq)→ NaNO3(aq)+ H2O(l)+ NH3(g)
When carbon dioxide is bubbled through aqueous solutions of the caustic alkalis, the carbonates are formed. With excess of the gas, the hydrogencarbonates are formed.
- 2NaOH(aq)+ CO2(g)→ Na2CO3(aq)+ H2O(l)
- Na2CO3(aq)+ H2O(l)+ CO2(g)→ 2NaHCO3(aq)
Chlorine reacts with excess of cold dilute caustic alkalis to form the hypochlorite, (NaClO or KClO).
- 2NaOH(aq)+ Cl2(g)→ NaCl(aq)+ NaClO(aq)+ H2O(l)
- 2KOH(aq)+ Cl2(g)→ KCl(aq)+ KClO(aq)+ H2O(l)
If excess chlorine is bubbled through hot concentrated solutions of caustic alkalis, the chlorates are formed, (NaClO3 or KClO3).
- 6NaOH(aq)+ 3Cl2(g)→ 5NaCl(aq)+ NaClO3(aq)+ 3H2O(l)
- 6KOH(aq)+ 3Cl2(g)→ 5KCl(aq)+ KClO3(aq)+ 3H2O(l)
Caustic alkalis are strong bases, which react with acids by neutralization reactions:
- NaOH(aq)+ HCl(aq)→ NaCl(aq)+ H2O(l)
- 2KOH(aq)+ H2SO4(aq)→ K2SO4(aq)+ 2H2O(l)
Chemical properties of Ca(OH)2
Action of heat
All hydroxides except potassium hydroxide (KOH) and sodium hydroxide (NaOH) decompose under the action heat to the oxide and steam. Calcium hydroxide undergoes thermal decomposition to calcium oxide and steam. Ca(OH)2(aq)→CaO(s)+ H2O(g)
Reaction with acids
Calcium hydroxide dissolves readily in dilute hydrochloric acid and nitric acid to form the corresponding calcium salts: Ca(OH)2(aq)+ 2HCl(aq)→CaCl2(aq)+ H2O(l) ;Ca(OH)2(aq)+ 2HNO3(aq)→Ca(NO3)2(aq)+ 2H2O(l)
Its reaction with dilute sulphuric acid is unsatisfactory due to the formation of calcium sulphate, which tends to precipitate on any dissolved lime.
Reaction with carbon dioxide
A solution of calcium hydroxide in water is called limewater. Carbon dioxide turns limewater “milky” due to the precipitation of white particles of calcium carbonate. Ca(OH)2(aq)+ CO2(g)→CaCO3(s)+ H2O(l)
Reaction with ammonium salts
Any ammonium salt will release ammonia gas when heated with calcium hydroxide solution. This is the common laboratory method for preparation of ammonia gas. Ca(OH)2(aq)+ 2NH4Cl(s)→2NH3(g)+ CaCl2(aq)+ 2H2O(l); Ionically; NH+4(s)+ OH-(s)→NH3(g)+ H2O(l)
Reaction with chlorine
- When chlorine gas is passed over moist cold solid calcium hydroxide, bleaching powder is formed. The formula of the bleaching powder is complex, and it is known with certainty but it probably contains Ca2+, Cl-and OH-ions and water. The reaction equation for its formation is usually written in “approximate” form: Ca(OH)2(s)+ Cl2(g)→ CaOCl2(s)+ H2O(l).The accepted but highly approximate formula of bleaching powder is Ca(OCl)2. It is known to contain a mixture of calcium hypochlorite, Ca(OCl)2, and basic calcium chloride, CaCl2.Ca(OH)2.H2O.
- When chlorine gas is bubbled in a cold milk of lime, a mixture of calcium chloride and calcium hypochlorite, Ca(OCl)2, is formed. 2Ca(OH)2(aq)+ 2Cl2(g)→ CaCl2(aq)+ Ca(OCl)2(aq)+ 2H2O(l)
- When chlorine is bubbled through hot milk of lime, calcium chloride and calcium chlorate are formed. 6Ca(OH)2(aq)+ 6Cl2(g)→ 5CaCl2(aq)+ Ca(ClO3)2(aq)+ 6H2O(l)
Reaction with hydrogencarbonates
Calcium hydroxide removes temporary hardness of water by precipitating the carbonate from the hydrogencarbonate. Ca(HCO3)2(aq)+ Ca(OH)2(aq)→2CaCO3(s)+ 2H2O(l)
The Uses of Metal Hydroxides
Describe the uses of metal hydroxides
Uses of NaOH
- Sodium hydroxide is used in the industrial manufacture of sodium, soaps and in the extraction of aluminium from bauxite.
- It is used in the manufacture of paper, dyes and bleach.
- Sodium hydroxide is a very common laboratory chemical. It is used in the qualitative as well as quantitative analysis.
- It is used in the preparation of insoluble metal hydroxides.
- It is used in the manufacture of artificial textile fibres.
- It is used to produce mineral salts by neutralization reactions with mineral acids.
Uses of KOH
- Potassium hydroxide is used in the manufacture of soft soap.
- Use as an electrolyte: aqueous potassium hydroxide is used as an electrolyte in alkaline batteries.
- Manufacture of biodiesel: potassium hydroxide works well in the manufacture of biodiesel by saponification of the fats in vegetable oil.4. Preparation of salts: many potassium salts are prepared by neutralization reactions involving potassium hydroxide.
Uses of Ca(OH)2
- Treatment of acid soils: Calcium hydroxide is a cheap alkali which can be used in large quantities to treat acid soils. The alkali neutralizes the excessive soil acidity, just like any alkali reacts with an acid, to form salt and water.
- Softening of hard water: Calcium hydroxide, in precisely calculated quantities, is used in the softening of temporary hard water as discussed early in chapter three. Ca(OH)2(aq)+ Ca(HCO3)2(aq)→ 2CaCO3(s)+ 2H2O(l)
- Preparation of mortar and whitewash: Mortar is prepared by mixing 1 part of slaked lime to 3 parts of sand into a paste with water. Slaked lime mixed with sand and water is used to stick bricks together.Whitewash is a thick suspension of slaked lime in water. It is smeared on the walls of buildings to give them a smooth protective finish.
- Manufacture of bleaching powder: Bleaching powder is manufactured by passing chlorine gas over moist calcium hydroxide as discussed previously in this chapter.
- Manufacture of paperFirst, a suspension of calcium hydroxide in water (milk of lime) is treated with sulphur dioxide gas to form calcium hydrogensulphite. Ca(OH)2(aq)+ 2SO2(g)→ Ca(HSO3)2(aq)Then, a solution of calcium hydrogen sulphite is used to remove the lignin from wood, leaving cellulose, which is used in paper manufacture. The action of removing lignin from wood is called bleaching of pulp.
- Manufacture of paints:Calcium hydroxide is used in the manufacture of undercoat paints which are applied as the first coat on plaster walls or on wood before applying the final gloss paint.
- Extraction of metals:Sodium hydroxide is used in the extraction of aluminium from bauxite ore.
- Liberation of ammonia: Calcium hydroxide is used in the Solvary Process to produce ammonia from ammonium chloride.
- Calcium hydroxide is a very important reagent inqualitative and quantitative analysis. It is used to determine the concentrations of acids in volumetric analysis, and in detection of ions present in unknown solutions in qualitative analysis.
Uses of Mg(OH)2
- Magnesium hydroxide is used as an antacid to neutralize stomach acid, and as a laxative. It is sold for medical use as chewable tablets, capsules, and as liquids having various added flavours. It is primarily used to alleviate constipation, but also relieve indigestion and heartburn.
- Magnesium hydroxide is also used as an antiperspirant and as armpit deodorant.
- Milk of magnesia (a suspension of magnesium hydroxide in water) is also applied and massaged onto the scalp, a few minutes before washing, to relieve symptoms ofseborrhoeaanddandruff. An additional use is for the treatment of acne or oily skin by applying topically, allowing to dry, and then washing it off the face (or other body part). It is also said to be used for seborrhoeaic dermatitis, which is a drying and flaking of the skin similar to dandruff but often occurring on the face.
- Magnesium hydroxide powder is used industrially as a non-hazardous alkali toneutralizeacidic waste waters.
- Solid magnesium hydroxide is used as fire and smoke retardant.
Carbonates and Hydrogen Carbonates
Preparation of Metal Carbonates and Hydrogen Carbonates by Different Methods
Prepare metal carbonate and hydrogen carbonates by different methods
here are many types and forms of metal carbonates in the earth’s crust. The most common and important carbonate is calcium carbonate which occurs naturally in the form of chalk, limestone or marble. Carbonates of other metals such as iron, copper, manganese, lead and zinc also occur naturally.Shells of snail, tortoise, fish and eggs are made of a great deal of carbonates.
There are four solid hydrogencarbonates. These are potassium, sodium, lithium and ammonium hydrogencarbonates. The hydrogencarbonates of calcium and magnesium occur in solution forms. Hydrogencarbonates are bases just like other ordinary carbonates.All metal hydrogencarbonates are soluble in water. Aluminium, zinc, iron, lead and copper hydrogencarbonates do not exist.
The method used to prepare carbonates depends on whether the carbonate is soluble in water or not. Sodium, potassium and ammonium carbonates are the only soluble metal carbonates.Solublecarbonates are prepared by passing carbon dioxide gas to the alkali. For example, sodium carbonate is formed when carbon dioxide gas is blown through sodium hydroxide solution.
CO2(g)+ 2NaOH(aq)→Na2CO3(aq)+ H2O(l)
If more carbon dioxide is bubbled through the solution, a second reaction occurs and sodium hydrogencarbonate is formed.Na2CO3(aq)+ H2O(l)+ CO2(g)→2NaHCO3(aq)
This is the easiest and convenient way for preparing hydrogencarbonates. Insoluble carbonates can be prepared by precipitation reactions. This involves adding a soluble carbonate to a solution of a salt of heavy metal. For example, when a solution of zinc carbonate is mixed with sodium carbonate solution, a precipitate of zinc carbonate is formed. ZnSO4(aq)+ Na2CO3(aq)→ZnCO3(s)+ Na2SO4(aq)
Likewise, copper carbonate can be precipitated by mixing copper (II) chloride solution with potassium carbonate solution.CuCl2(aq)+ K2CO3(aq)→CuCO3(s)+ 2KCl(aq)
Classification ofMetal Carbonates
Classify metal carbonates
Metal carbonates are classified based on their solubility in water. Classified on this basis, we have soluble and insoluble carbonates. Potassium, sodium and ammonium carbonates are soluble in water. All other carbonates are insoluble in water.
The Chemical Properties of Metal Carbonates
Analyse the chemical properties of metal carbonates
Reaction with acids
Carbonates and hydrogencarbonates react with dilute acids to produce carbon dioxide, salt and water. For example:
CaCO3(s)+ 2HCl(aq)→CO2(g)+ CaCl2(aq)+ H2O(l)
2NaHCO3(aq)+ H2SO4(aq)→ 2CO2(g)+ Na2SO4(aq)+ 2H2O
Action of heat
Excluding sodium and potassium carbonates, all other carbonates decompose on heating to form oxides of corresponding metals and carbon dioxide. For example:PbCO3(s)→PbO(s)+ CO2(g)
A hydrogencarbonate decomposes to give a carbonate, carbon dioxide and water.2NaHCO3(aq)+ Na2CO3(s)→H2O(l)+CO2(g)
Distinction between carbonates and hydrogencarbonates
We can easily distinguish a carbonate from a hydrogencarbonate by the use of magnesium sulphate.When a carbonate solution is added to a magnesium sulphate solution, a white precipitate of magnesium carbonate is formed:Na2CO3(aq)+ MgSO4(aq)→MgCO3(s)+ Na2SO4(aq)
When a similar reaction is performed with a hydrogencarbonate solution, no white precipitate is formed:2NaHCO3(aq)+ MgSO4(aq)→Mg(HCO3)2(aq)+ Na2SO4(aq)
This is because magnesium hydrogencarbonate is soluble in water. However, when the solution is boiled, magnesium hydrogencarbonate decomposes to give a white precipitate of magnesium carbonate.Mg(HCO3)2(aq)→MgCO3(s)+ H2O(l)CO2(g)
The Uses of Carbonates and Hydrogen Carbonates
Describe the uses of carbonates and hydrogen carbonates
For easy understanding of the uses of carbonates and hydrogencarbonates, each of the most common of these compounds will be dealt with separately.
Uses of sodium carbonate, Na2CO3
- It is widely used in the manufacture of sodium silicate, which is used to manufacture glass (for more details, and the reaction equations involved, refer to the uses of calcium carbonate).
- It is used to manufacture soap and paper.
- Manufacture of sodium hydroxide: sodium hydroxide is prepared by adding slaked lime (calcium hydroxide) to sodium carbonate and the mixture continuously stirred. A precipitate of calcium carbonate forms and is filtered off: Na2CO3(aq)+ Ca(OH)2(s)→ 2NaOH(aq)+ CaCO3(s).The filtrate, which is a solution of sodium hydroxide, is then evaporated to obtain crystals of sodium hydroxide.
- It is used in volumetric analysis to determine the concentration of acids (standardize acids).
- Sodium carbonate is used for softening hard water.
- It is used in medicine as an antacid.
- It is also important inphotographyand the textile industry (where itis used to facilitate the chemical bonding between the dye and the fibre).
- Manufacture of ‘water glass’: When sodium carbonate is heated together with silicon dioxide (SiO2), sodium silicate (NaSiO3) and carbon dioxide are produced. Na2CO3(s)+ SiO2(s)→ NaSiO3(s)+ CO2(g).A concentrated solution of sodium silicate in water, known as water glass is used as a preservative for eggs. It is also used asan adhesive in paper making and in television tubes.
Uses of sodium hydrogencarbonate, NaHCO3
This is the most important hydrogencarbonate. The following are some of its uses:
- Health salt: The salt finds a variety of medicinal uses, particularly in stopping diarrhoea and, as anantacidto treat heartburn, indigestion, and other stomach disorders. It is also used to treat various kidney disorders and to increase the effectiveness of sulphonamides.A heath salt is a mixture of sodium hydrogencarbonate, tartaric acid and sodium potassium tartrate.Acting as antacid, sodium hydrogencarbonate neutralizes the hydrochloric acid produced in the stomach. Because it is very soluble, it acts very fast thus providing a quick relief for symptoms caused by excess acid in the stomach.
- It is used in the removal of grease from clothes and other articles. Most of the cleaning agents that are used for the removal of stains tend to contain a small amount of sodium bicarbonate as a very important ingredient.
- Use in industry: sodium bicarbonate is used in textile industry for dyeing and printing operations; in leather industry as a neutralizer of dyeing agents in tanning processes; and in rubber and plastic industry as a blowing agent, as it releases carbon dioxide which is used to shape the object that is made from the rubber and plastic.
- Baking: sodium bicarbonate is widely used to bake breads and cakes. The baking soda contains a large quantity of sodium hydrogencarbonate and is always accompanied by a small quantity of acid phosphate. When baking soda mixed with dough is heated, the sodium hydrogencarbonate decomposes to give bubbles of carbon dioxide which make the dough rise: 2NaHCO3(s)→ Na2CO3(s)+ CO2(g)+ H2O(l).Commercial baking powder is a mixture of baking soda and organic acids such as citric or tartaric acid.
Uses of calcium carbonate, CaCO3
Remember we learned early that calcium carbonate exists in three forms: chalk, limestone and marble. So, in this context, the uses of three forms will be discussed.
- Sodium carbonate is used in the blast furnace for extraction of iron from its ore.
- It is used in the manufacture of glass. Heating a mixture of calcium carbonate (CaCO3), sodium carbonate (Na2CO3) and sand (SiO2) very strongly, at a temperature of 1300 to 1400°C, produces a clear melt. When this melt is cooled down, it hardness to form glass.Upon heating these compounds, carbon dioxide is given off, leaving a mixture of sodium silicate and calcium silicate with excess silicon dioxide: CaCO3(s)+ SiO2(s)→ CaSiO3(s)+ CO2(g)Na2CO3(s)+ SiO2(s)→ Na2SiO3(s)+ CO2(g).Glass is a mixture of sodium and calcium silicates.
- Limestone is used to make cement. Limestone powder and clay are roasted and the product is grinded to form a fine powder called cement.
- Calcium carbonate is used in the manufacture of lime, which is used as a fertilizer.
- Marble is used on walls of houses and other buildings to make them look attractive.
- Limestone is used in road construction. Small pieces of marble are stuck together and then covered with tar to gives a smooth hard surface, which can last for several years.
- A mixture of limestone and linseed oil is used as putty, which helps in retaining the structure of windows, doors and other wooden structures in position in houses and buildings.
- Calcium carbonate, in the fine precipitate form, can be used in the manufacture of toothpaste.
Nitrates
Preparation of Metal Nitrates
Prepare metal nitrates
Nitrates are important compounds widely known for industrial purposes. Sodium nitrate has been known for quite a long time. It occurs in nature as saltpetre (NaNO3).
Nitrates are usually prepared by methods which involve crystallization. This can be done by reacting nitric acid with metals, oxides, hydroxides or carbonates.
- In the laboratory, sodium nitrate may be prepared by neutralizing sodium hydroxide solution with nitric acid.NaOH(aq)+ HNO3(aq)→ NaNO3(aq)+ H2O(l)
- Calcium nitrate may be made in the laboratory by the action of nitric acid upon calcium carbonate.CaCO3(s)+ 2HNO3(aq)→ Ca(NO3)2(aq)+ H2O(l)+ CO2(g)
- Ammonium nitrate can be made by neutralization of ammonia solution with nitric acid.NH3(aq)+ HNO3(aq)→ NH4NO3(aq)
- Lead nitrate can be obtained by the reaction between lead oxide and dilute nitric acid.PbO(s)+ 2HNO3(aq)→ Pb(NO3)2(aq)+ H2O(l)
- Copper nitrate can be prepared by dissolving copper oxide in dilute nitric acid.CuO(s)+ 2HNO3(aq)→ Cu(NO3)2(aq)+ H2O(l)
- Nitrates of certain metals can be prepared by reacting the metals with dilute or concentrated nitric acid.3Pb(s)+ 8HNO3(aq)→ 3Pb(NO3)2(aq)+ 4H2O(l)+ 2NO(g);3Cu(s)+ 8HNO3(aq)→ 3Cu(NO3)2(aq)+ 4H2O(l)+ 2NO(g)
However, the nitrates of common heavy metals, except lead nitrate, are very soluble in water and they are deliquescent. This makes it a matter of grater difficult to prepare their crystals. All the crystals are white in colour except those of copper (II) nitrate, which are blue. All nitrates are soluble in water.
The Chemical Properties of Metal Nitrates
Explain the chemical properties of metal nitrates
Action of heat
The chemical properties of metal nitrates vary according to the position of the metal in the reactivity series. Metal nitrates give a variety of products when thermally decomposed. This fact is summarized below:

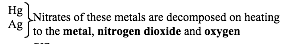
Ammonium nitrate is decomposed by heat into dinitrogen oxide and water: NH4NO3(s)→N2O(g)+2H2O(l)
The brown ring test
All nitrates undergo the same reaction with iron (II) sulphate and concentrated sulphuric acid and this reaction becomes a test for the soluble nitrates, the brown ring test. This test is carried out by crushing a little potassium nitrate, putting it in a test tube, adding water to a depth of about 2 cm and then shaking the test tube to dissolve the potassium nitrate (note that any metal nitrate could have been used instead of potassium nitrate). This is followed by adding a little sulphuric acid and then two or three crystals of iron (II) sulphate, which have also been crushed. The contents are shaken to dissolve them. Finally, the test tube is held in a slanting position and a slow continuous stream of concentrated sulphuric acid is poured down the side of the test tube. The acid forms a separate layer underneath the aqueous layer and, at the junction of the two, a brown ring will be seen. This brown ring is the characteristic test for all soluble nitrates.
Explanation
- The concentrated sulphuric acid and the nitrate react to produce nitric acid:KNO3(s)+ H2SO4(aq)→ KHSO4(aq)+ HNO3(aq).
- The nitric acid formed is reduced by some of the iron (II) sulphate to nitrogen monoxide, NO:6FeSO4(s)+2HNO3(aq)+3H2SO4(aq)→3Fe2(SO4)3(aq)+4H2O(l)+2NO(g)
- The nitrogen monoxide produced then reacts with some of the remaining iron (II) sulphate to form a dark brown complex, FeSO4.NO, which appears as a ring. FeSO4(aq)+ NO(g)→FeSO4.NO(aq)
The Uses of Metal Nitrates
Explain the uses of metal nitrates
Uses of Metal Nitrates include:
- Potassium nitrate is used in the preparation of gunpowder (mixture of charcoal, sulphur and potassium nitrate) and other explosives. When gunpowder is ignited, it explodes. In addition, ammonium nitrate is also used in making explosives and blasting agents which are used in mines and quarries.
- Food preservation: nitrates and nitrites are used in curing (salting and pickling) meats and fish. Not only do they kill bacteria but they also produce a characteristic flavour, and give meat a red or pink colour. Sodium nitrate or potassium nitrate is used as a source of nitrite (nitrogen dioxide), NO2. The nitrite breaks down in the meat into nitric oxide (nitrogen monoxide), NO which helps to prevent oxidation.
- Manufacture of fertilizers: nitrogenous fertilizers are mainly nitrates. They include ammonium nitrate, potassium nitrate, sodium nitrate and calcium nitrate. Nitrogenous fertilizers are manufactured form nitric acid.
- Silver nitrate is used in photography, silvering mirrors, making marking ink, etc.
- Antiseptics: antiseptics are chemical agents that are used on the skin and mucous membranes to kill germs. Silver compounds such as silver nitrate and sulphadiazine have been used to prevent the infection of burns and some eye infections and to destroy warts.
Chlorides
Preparation of Metal Chlorides by Direct and Indirect Methods
Prepare metal chlorides by direct and indirect methods
In everyday life, we come across many chlorides. Sodium chloride, literally known as table salt, is the commonest chloride that we use in every walk of our lives. It is added to some foods to make them taste better. In Tanzania, the salt is found in large deposits at Uvinza in Kigoma region. The salty taste of sea water is mainly due to dissolved sodium and potassium chlorides.
Metal chlorides can be prepared by direct and indirect methods. All metals are attacked by chlorine to form chlorides. Metals above hydrogen in the reactivity series can displace hydrogen of the hydrochloric acid to form metal chlorides. Hydroxides, oxides or carbonates of potassium, sodium or calcium react with dilute hydrochloric acid to produce chlorides of respective metals. When dilute hydrochloric acid is added to the aqueous salts of lead and silver, they produce lead and silver chlorides respectively. These are the only two common insoluble chlorides. The rest of the chlorides are all soluble in water.
Preparation of chlorides by direct method
Metal chlorides can be prepared by direct action of chlorine gas on metals. For example, iron (III) chloride is made by the action of chlorine on iron. Figure 9.2 shows how iron (III) chloride can be prepared by passing chlorine directly over a heated metal. An iron wire is placed in a hard glass tube as shown and a stream of pure chlorine gas is passed over it. When the wire is heated, by means of a burner, the wire glows and the source of heat is removed. The reaction continues even without further application of heat. Black crystals of iron (III) chloride are collected in the small bottle, which acts as a condenser.2Fe(s)+ 3Cl2(g)→2FeCl3(s)
Because anhydrous iron (III) chloride is deliquescent, it should be removed and placed in a desiccator. Note: When hydrogen chloride gas is used instead of chlorine, iron (II) is produced.Fe(s)+ 2HCl(g)→FeCl2(s)+ H2(g)
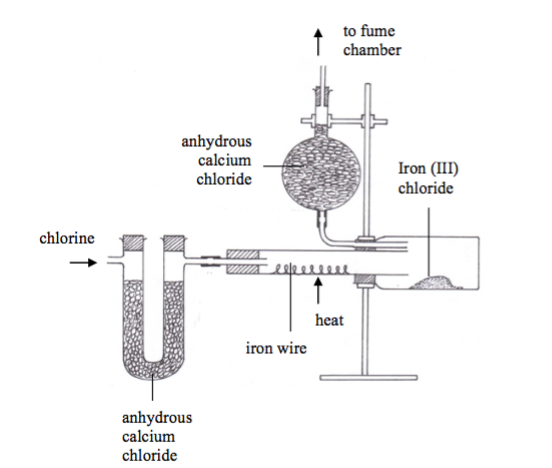
A similar procedure above can be used for preparation of aluminium (III) chloride. 2Al(s)+ 3Cl2(g)→2AlCl3(s)
Preparation of soluble chlorides
Soluble chlorides may be prepared by the action of dilute hydrochloric acid on (i) oxides, (ii) hydroxides, (iii) carbonates, or (iv) metals
- MgO(s)+ 2HCl(aq)→ MgCl2(aq)+ H2O(l)
- KOH(aq)+ HCl(aq)→ KCl(aq)+ H2O(l)
- CaCO3(s)+ 2HCl(aq)→CaCl2(aq)+ H2O(l)+ CO2(g)
- Zn(s)+ 2HCl(aq)→ ZnCl2(aq)+ H2(g)
Methods of preparation of soluble chlorides can be summarized as shown below:

Preparation of insoluble chlorides
- Lead (II) chloride, PbCl2:This is a white insoluble substance prepared by the reaction of a solution of any soluble lead (II) salt with a solution of any soluble chloride, e.g.Pb(NO3)2(aq)+ 2HCl(aq)→PbCl2(s)+ 2HNO3(aq)
- Silver chloride, AgCl:This is a white insoluble compound prepared by adding a solution of any soluble silver salt to a solution of any soluble chloride, e.g.AgNO3(aq)+ NaCl(aq)→ AgCl(s)NaNO3(aq)This is a soluble decomposition reaction.
The Chemical Properties of Metal Chlorides
Explain the chemical properties of metal chlorides
Chemical properties of metal chlorides include:
- Metal chlorides liberate hydrogen chloride gas when warmed together with concentrated sulphuric acid, e.g.2NaCl(s)+ H2SO4(aq)→ Na2SO4(aq)+ 2HCl(g)
- When concentrated sulphuric acid is added to a mixture of a chloride and an oxidizing agent, and then warmed, a greenish gas, chlorine is evolved.2NaCl(s)+ 2H2SO4(aq)+ MnO2(s)→ MnO4(aq)+ Na2SO4(aq)+ 2H2O(l)+ Cl2(g)
- Several chlorides are easily hydrolyzed by water, e.g. magnesium, zinc and iron chlorides.If solutions of the chlorides are evaporated, basic salts or oxides of metals remain.
- Most chlorides are more volatile than most salts, a property which makes them suitable for use in “flame test” in which certain metals can be detected by the colour their vapour imparts to the Bunsen flame.5. When heated, ammonium chloride sublimes forming ammonia gas and hydrogen chloride gas.NH4Cl(s)NH3(g)+ HCl(g)
- Hydrated chlorides give their water of crystallization when heated.MgCl2.6H2O(s)→ MgCl2(s)+ 6H2O(g)
- Soluble chlorides react with silver nitrate or lead nitrate to form insoluble white precipitate of silver and lead chlorides respectively.
The Uses of Metal Chloride
Explain the uses of metal chloride
Sodium chloride, NaCl
- Sodium chloride is used a precursor or a starting point in the manufacture of other important chemicals such as sodium hydroxide, sodium carbonate, sodium hydrogencarbonate, sodium sulphate, sodium carbonate decahydrate and other sodium compounds.
- It is used in the manufacture of soaps: In the industrial manufacture of soap, tallow (fat from animals such cattle and sheep) or vegetable fat is heated with sodium hydroxide through a process called saponification. Once the saponification reaction is over, sodium chloride is added to precipitate the soap.
- As a chloride, it yields hydrochloric acid and chlorine gas, which is used as bleaching agent, and in the manufacture of hypochlorite solutions.
- Common salt is used to add flavour to our food at home.
- It is used as a food preservative by bringing the dehydration effect to bacteria. It is an important preservative used in the preservation of cheese, dairy products, meat, pickles and sauces.
- Brine (concentrated sodium chloride solution) is used for extraction of sodium metal by electrolytic method. Chlorine gas and sodium hydroxide are the major by-products from the electrolysis of brine.
- Melting ice: sodium chloride has a property of lowering the melting point of ice. Therefore, it is spread on icy roads during winter to quicken the melting of ice.
- It is used in glazing (shiny and smooth finishing) pottery
- Cleansing agent: sodium chloride has also been used as a cleansing agent since long time. In ancient times, it was used for household cleaning simply by rubbing it against surfaces. It is also an ingredient of soaps, detergents and shampoos.
Ammonium chloride, NH4Cl
- Ammonium chloride is used as a constituent of the Lenclanche’ voltaic cell.
- Ammonium chloride is sold in blocks for use in cleaning the tip of soldering iron and can also be included in solder as flux.
- Other uses of ammonium chloride include use as a feed supplement in cattle, in hair shampoo, in textile printing, in the glue that binds plywood, as an ingredient in nutritive media for yeast, in cleaning products, and as a cough medicine - its expectorant action is caused by irritative action on the bronchial mucosa. This causes the production of excess respiratory tract fluid, which presumably is easier to cough up.
- Its biological applications include using it as an energy source for microbiological growth of organisms.
Aluminium chloride, AlCl3
- Aluminium chloride is used for petroleum refining. It is also used in the manufacture of synthetic lubricating oils.
- It is used for manufacturing of paints, detergents and has also been used as an antiperspirant.
- It is used for production of synthetic rubber.
Calcium chloride, CaCl2
- Calcium chloride is used mainly as a drying agent for most gases (except ammonia, with which it forms a compound). This is because calcium chloride is a deliquescent salt. It tends to absorb moisture from gases.
- Like sodium chloride, it is spread on icy roads in winter to help melt the ice. Calcium chloride melts the ice faster than any other chemical compound.
- Calcium chloride has a salty taste and is used as the main ingredient in many types of food items including snacks.
- Calcium chloride prevents spoilage of food and is popularly used as a preservative in packed foods. It also helps to keep the food healthy and fresh for a longer duration.
- Being strongly hygroscopic, a layer of calcium chloride is applied on roads and in mines to minimize dust problems.
Magnesium chloride, MgCl2
- Magnesium chloride is used to lubricate cotton threads in the spinning industry.
- Dentistry: magnesium chloride is a constituent of the cement used to fill cavities in teeth.
Potassium chloride, KCl
- The majority of potassium chloride is used for making fertilizers (muriate of potash) since the growth of many plants is limited by their potassium uptake.
- As a chemical feedstock, it is used for the manufacture of potassium hydroxide and potassium metal.
- It is also used in medicine, scientific applications and in food processing.
- Along with sodium chloride and lithium chloride, potassium chloride is used as a flux for the gas welding of aluminium.
- It is widely used as an additive in the paper industry and in the manufacture of dyes.
Sulphates
Preparation of Soluble and Insoluble Sulphates
Prepare soluble and insoluble sulphates
Metal sulphates are generally soluble in water, except for the commonly known insoluble sulphates of lead (PbSO4) and barium (BaSO4). Calcium sulphate is sparingly soluble.The sulphate of alkali metals and those of the alkaline earth metals (Mg, Ca, Sr and Ba) are very stable to heat.
Soluble sulphates can be prepared in the laboratory by reacting metals, oxides, hydroxides or carbonates with dilute sulphuric acid and then isolating the crystals. The sulphates are isolated by the usual methods of preparing soluble salts.
- Fe(s)+ H2SO4(aq)→ FeSO4(aq)+ H2(g)
- MgO(s)+ H2SO4(aq)→ MgSO4(aq)+ H2O(l)
- 2NaOH(aq)+ H2SO4(aq)→ Na2SO4(aq)+ 2H2O(l)(d)
- CaCO3(s)+ H2SO4(aq)→ CaSO4(aq)+ H2O(l)+ CO2(g)
Preparation of insoluble sulphates
The best method to prepare insoluble metal sulphates is by precipitation reactions. This is achieved by reacting their soluble salts with dilute sulphuric acid:
- BaCl2(aq)+ H2SO4(aq)→ BaSO4(s)+ 2HCl(aq)
- Pb(NO3)2(aq)+ H2SO4(aq)→PbSO4(s)+ 2HNO3(aq)
The sulphates are isolated by the usual methods of preparing insoluble salts.
Chemical Properties of Sulphates
Explain chemical properties of sulphates
Sulphates of all metals are normal salts and have the following chemical properties:
- All sulphates give a white precipitate when treated with aqueous salts of lead and barium, e.g. BaCl2(aq)+ K2SO4(aq)→ BaSO4(s)+ 2KCl(aq);Na2SO4(aq)+Pb(NO3)2(aq)→ PbSO4(s)+ 2NaNO3(aq)
- Barium and lead sulphates are the only two common insoluble sulphates. Calcium sulphate is sparingly soluble. The rest of the sulphates are soluble in water.
- Sulphates of the metals in group I and II of the periodic table are very stable to heat. Strong heating decomposes some of the sulphates of the heavier metals.Iron (II) sulphate disproportionates when heated: 2FeSO4(s)→ Fe2O3(s)+ SO2(g)+ SO3(g)Iron (III) sulphate gives a good yield of sulphur trioxide gas: Fe2(SO4)3(s)→ Fe2O3(s)+ 3SO3(g)
- Hydrated sulphates decompose on heating to form oxides, water and sulphur trioxide. CuSO4.5H2O(s)→ CuO(s)+ 5H2O(g)+ SO3(g);FeSO4.7H2O(s)→ FeO(s)+ 7H2O(g)+ SO3(g).But hydrated sodium sulphates is stable to heat.It only loses its water of crystallization when heated. Na2SO4.10H2O(s)→ Na2SO4(s)+ 10H2O(g)
Uses of Sulphates
Describe uses of sulphates
Sulphates are salts of considerable importance in our everyday lives. The following are the uses of some sulphates:
Sodium sulphate (Na2SO4.10H2O), also known as Glauber’s salt.
It is used as a mild purgative in medicine. The anhydrous salt, Na2SO4,is used as a laxative. It also finds its use in the manufacture of glass.
Calcium sulphate
In the form of Plaster of Paris (CaSO4.½H2O), is used to make plaster casts that are used in hospitals for the repair of broken limbs. When in the form of gypsum (CaSO4.2H2O), it is used in the manufacture of cement, moulds, wall plasters and wallboards, and inexpensive art objects. Among the many other uses of calcium sulphate are its uses as a pigment in white paints, coating agent in papers, in the manufacture of sulphuric acid and sulphur, and as a drying agent in many laboratory and commercial purposes.
Some uses of aluminium sulphate are as follows:
- It is used in paper making where it binds the paper fibres together.
- It is also used in the manufacture of aluminium hydroxide, which is used for mordant dyeing. The aluminium hydroxide formed by the hydrolysis of the sulphate is deposited on the cloth fibres, where it helps the dye to stick well on the fibre. The salt is also used in paper sizing (i.e. giving it body and strength), and waterproofing the cloth.
- Aluminium sulphate is an important chemical in the treatment of urban water. It precipitates colloidal matter from water. Microorganisms e.g. bacteria and algae are also captured during the coagulation process and precipitated with mud.
- Aluminium sulphate is used in the “foam” type fire extinguishers. The sulphate is mixed with sodium carbonate or hydrogencarbonate to produce carbon dioxide and aluminium (III) hydroxide, Al(OH)3, which mix together to formthe foam. The foam is effective in excluding air from oil fires hence helping to put the fire off.
- Aluminium sulphate is used in the tanning of leather. It is also used as a fertilizer.
Iron (II) sulphate is used:
- in the manufacture of ink and dye
- in tanning leather (iron-tanning)
- to make tablets prescribed to patients with iron deficiency.
- to prepare a reddish-brown iron (III) oxide (‘red oxide’) which is used as a pigment.
- as a weed killer ( herbicide) and as a fungicide.
- for treating sewage and water.
- to coagulate ( bind together) blood in slaughterhouses.
Copper (II) sulphate finds many uses which include:
- Manufacture of copper fungicides (CuSO4.5H2O) such as red and blue copper, which is sprayed on crops to prevent certain species of fungi.
- Manufacture of certain green pigments.
- Copper (II) sulphates is used for makingwashes such as a “Bordeaux mixture”, used in sprayingvines and potatoes to kill moulds which would otherwise injure the plants.
- Manufacture of insecticides such as copper arsenite and Paris green for control of fungus diseases.
- Correction of copper deficiency in soils and in animals
- It is used as a growth stimulant in pigs and broiler chickens.
- It is also used as a molluscide for the destruction of slugs and snails, particularly the snail, which is a host of liver fluke.
- Copper sulphate is used as a timber preservative for the prevention of wood rot.
- It is used as an electrolyte in copper-plating and as a catalyst in preparation of ethanol.
Zinc sulphate has the following uses:
- it is used as an emetic and for treatment of certain skin diseases.
- Hydrated zinc sulphate (ZnSO4.7H2O) is used as an agent in printing and textile dyeing, as an antiseptic and in preserving wood and hides. It is also in zinc-plating by electrolysis.
- It is used as an antibacterial treatment for sewage, a miticide and an herbicide.
- It is used as a component of cosmetics (such as skin fresheners) and an ingredient in some deodorants.
- Diluted “White Vitriol”, (ZnSO4.7H2O), is used in medicine in the preparation of eye lotions and mouth washes. It is also used to assists the healing of wounds.
- Ammonium sulphate is used as a fertilizer.
- Magnesium sulphate, in the form of Epsom salt (MgSO4.7H2O), is used as a mild purgative.
- Barium sulphate (BaSO4) is used in the manufacture of white pigments, in white paints.
- The alums are used in dyes and in leather industry. Alums are double salts of general formula, X2SO4.Y2(SO4)3.24H2O, whereX is Na, K or NH4and Y is Fe(III), Al or Cr. The two commonest alums are: Potash alum, K2SO4.Al2(SO4)3.24H2O (colourless); and; Iron (III) alum, (NH4)2SO4.Fe2(SO4)3.24H2O(purple).






EmoticonEmoticon