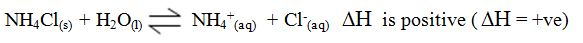JOIN US WHATSAPP
CLICK HERE
JOIN US TELEGRAM
CLICK HERE
TOPIC 7: CHEMICAL KINETICS, EQUILIBRIUM AND ENERGETICS
The Rate of Chemical Reactions
Comparison between the Rates of Chemical Reactions
Compare the rates of chemical reactions
Chemical reactions take place at different rates. Some are fast whereas others are very slow. Let us consider the following reactions:
- Addition of sodium metal to water: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) The reaction takes place immediately and violently. It is therefore a fast reaction.
- The rusting of iron in the presence of air and water giving hydrated iron (III) oxide, F2O3.XH2O: This is an extremely slow reaction.
These two reactions could be taken as representative examples of extremely fast and extremely slow reactions, respectively.There are, however, other reactions which proceed at rates intermediate between these two extremes. Rates of some of these reactions can be measured.
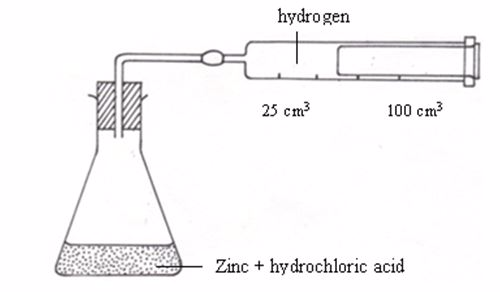
The rate of a chemical reaction can be measured in various ways. Let us consider the reaction between zinc and sulphuric acid to produce zinc sulphate and hydrogen gas:
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
When zinc is added to dilute sulphuric acid in a flask, they react together. The zinc slowly disappears and the gas (H2) bubbles off. After sometime, the bubbles of a gas form less quickly. The reaction is slowing down. Finally, no more bubbles appear. The reaction is over, because all the acid has been used up. Some zinc remains behind in a beaker.
In this reaction both zinc and sulphuric acid get used up in the reaction. At the same time, zinc sulphate and hydrogen form. The rate of this reaction could be determined by measuring any of the following:
- the amount of zinc used up per unit of time;
- the amount of sulphuric acid used up per unit of time;
- the amount of zinc sulphate produced per unit of time; or
- the amount of hydrogen produced per unit of time.
In general, the rate of a chemical reaction is determined by measuring the amount of reactant used up per unit of time or the amount of product produced per unit of time. Therefore, the rate of a chemical reaction simply refers to the amount of reaction which occurs in a unit time.
Experiments to Measure the Rates of Chemical Reactions
Perform experiments to measure the rates of chemical reactions
For the reaction described above, it is easiest to measure the amount of hydrogen produced per minute. The hydrogen can be collected as it bubbles off and its volume can then be measured as shown in figure

Apparatus for measuring the production of gas
An experiment may be designed to measure the volume of hydrogen produced after every twenty seconds or so and then recording the data in a notebook.The table below shows sample results from such an experiment.
| Time (s) | Volume of hydrogen gas (cm3) |
| 0 | 0 |
| 20 | 13 |
| 40 | 22 |
| 60 | 30 |
| 80 | 37 |
| 100 | 41 |
| 120 | 44 |
| 140 | 46 |
| 160 | 47 |
| 180 | 47 |
| 200 | 47 |
Questions from the experiment
Use these data to draw a graph of time (horizontal axis) against volume of hydrogen (vertical axis).
There are also other ways by which rates of chemical reactions can be measured. These include measuring the:
- change in intensity of colour: Many chemical reactions involve a change in colour. Potassium permanganate, for example, when it reacts with sulphur dioxide it changes from purple to colourless. The rate of such a reaction could be determined by measuring the rate at which the colour changes.
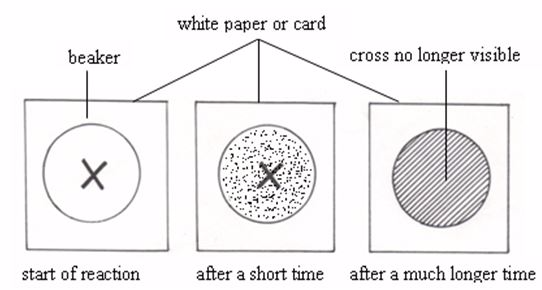
- formation or disappearance of a precipitate: The reaction between hydrochloric acid and sodium thiosulphate produce a yellow precipitate of sulphur. The rate at which this precipitate forms is a measure of the rate of a reaction.
Factors Affecting the Rate of Chemical Reactions
Reversible and Irreversible Reactions
Equilibrium Reactions
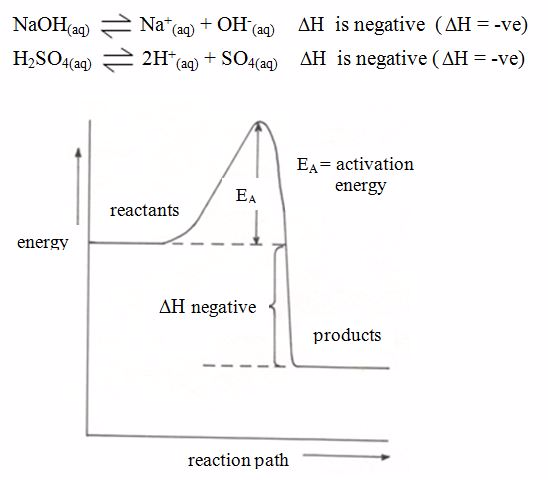
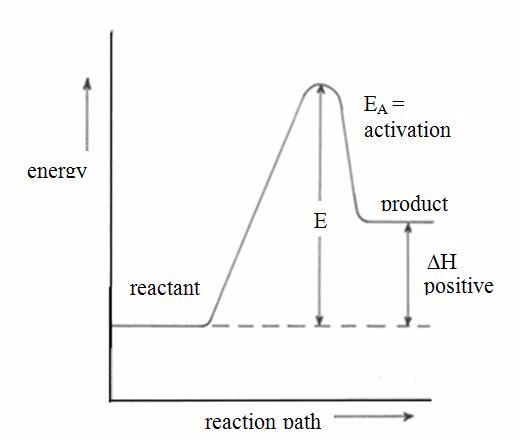
Endothermic and Exothermic Reaction