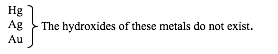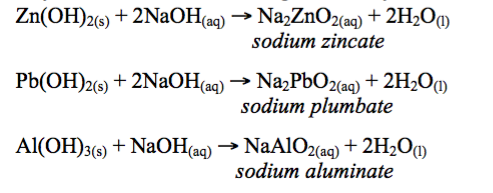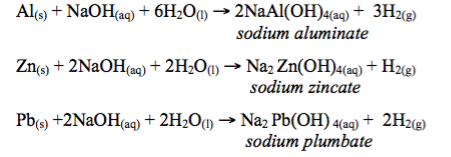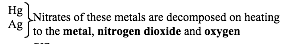JOIN US WHATSAPP
CLICK HERE
JOIN US TELEGRAM
CLICK HERE
TOPIC 9: COMPOUNDS OF METALS
Metals combine with other substances to form compounds. There is a diversity of metal compounds known. One can make an endless list of the compounds of the metals. In this chapter, we are going to concentrate our efforts on the following compounds of the metals: oxides, hydroxides, carbonates and hydrogencarbonates, nitrates, chlorides and sulphates.
Oxides
Preparation of Oxides of Some Metals by Direct and Indirect Methods
Prepare oxides of some metals by direct and indirect methods
All elements except helium, neon and argon form compounds with oxygen. This is because oxygen is quite reactive. Binary compounds of oxygen are known as oxides. Therefore, a metal oxide is a binary compound of oxygen and a metal.
Metal Oxides
Classify metal oxides
Chemical properties of metal oxides
- Reaction with water: The oxides of potassium, sodium and calcium are very soluble in water. They will react vigorously with cold water to produce the corresponding hydroxides. The oxides of metals below calcium in the reactivity series are all insoluble in water.
- Reaction with acids: The oxides of metals above hydrogen in the reactivity series react with dilute acids to produce salt and water.
- Reaction with alkalis: Some oxides react with alkalis to produce salt and water. The oxides of this nature include ZnO, Al2O3, PbO and SnO.
The Reactions of Metal Oxides with Water and Dilute Acids
Demonstrate the reactions of metal oxides with water and dilute acids
Preparation of metal oxides
Metal oxides can be prepared by:
- direct methods. This involves heating metals directly in air.
- indirect methods. This involves such methods like heating carbonates and hydrogencarbonates of certain metals in air, and reacting certain metals with certain acids.
Preparation of metal oxides by direct combination(Direct method)
In this method, oxides can be prepared by direct combination of metals with oxygen. This involves heating a metal in air. When some metals are burned in the air, they react with oxygen of the air to form metal oxides. However, this method is not intensively used because some metals tend to form a protective layer of an oxide on the surface of metal and prevent further attack by oxygen. The best example of such metals is aluminium which, when heated in the air, forms a protective layer of aluminium (III) oxide (Al2O3) on the surface of a metal whichprevents further attack by oxygen. Table 9.1 shows the products formed when certain metals are burned in the air.
Table 9.1: The reaction of metals with oxygen
| Metal | How it reacts | Product |
| Barium | burns with a green flame | white solid (barium (II) oxide, BaO) |
| Calcium | burns with a brick-red flame | white solid (calcium oxide, CaO) |
| Sodium | burns with a yellow flame | white solid (sodium oxide, Na2O) |
| Potassium | burns with a purple flame | white solid (potassium oxide, K2O) |
| Magnesium | burns with a white flame | white solid (magnesium oxide, MgO) |
| Iron | burns with yellow sparks | Blue-black solid (iron (II) oxide, FeO) |
| Copper | doesnot burn, turns black | black solid (copper (II) oxide, CuO) |
Preparation of metal oxides by indirect methods
This method involves thermal decomposition of salts. When some salts are heated, they decompose into oxides and other products as well. If the anion part of the salt heated contains some oxygen, a portion of this oxygen may remain bonded to the central metal atom.
However, this method is limited only to those compounds of metals below sodium in the electrochemical series. For instance, potassium oxide or sodium oxide cannot be prepared by action of heat on their carbonates. Thermal decomposition of some metals is as shown by the following equations:
- CuCO3(s)→CuO(s)+ O2(g)
- CaCO3(s)→CaO(s)+ CO2(g)
- ZnCO3(s)→ ZnO(s)+ CO2(g)
Hydroxides also behave in a similar manner. When hydroxides of metals below sodium in the electrochemical series are heated, they decompose into respective oxides, giving off water in the form of steam.
- Ca(OH)2(s)→ CaO(s)+ H2O(g)
- Mg(OH)2(s)→ MgO(s)+ H2O(g)
The oxides can also prepared by heating some nitrates and sulphates:
- 2Pb(NO3)2(s)→ 2PbO(s)+ 4NO2(g)+ O2(g)
- 2Cu(NO3)2(s)→ 2CuO(s)+ 4NO2(g)+ O2(g)
Nitrates of silver and mercury are not suitable for preparation of oxides by thermal decomposition because they decompose to metals directly when heated. The bond between oxygen and a metal atom is not strong enough to withstand the thermal energy:
- 2AgNO3(s)→ 2Ag(s)+ 2NO2(g)+ O2(g)
The Uses of Metal Oxides
Explain the uses of metal oxides
Metal oxides find a wide range of uses. The following are the uses of the most common oxides:
Uses of calcium oxide (CaO)
- Making mortar:Calcium oxide reacts with water to form the hydroxide, Ca(OH)2, known as slaked lime.Mortar is made by mixing slaked lime, sand and water, and is used in sticking bricks together and in forming smooth surfaces on walls of buildings.
- Calcium oxide is used for making whitewash, which is used in marking sport’s fields, roads and is brushed on walls of buildings to give them the white colour. Whitewash is a suspension of slaked lime in water.
- Cement and concrete: Cement is made by heating together lime or limestone and clay. The product is a mixture of calcium silicates and aluminates. Clay is hydrated aluminium silicate. A mixture of cement, sand, stones and water gives concrete, which on setting becomes extremely hard. It is the materials used for making foundations of buildings, pillars, roads, paths, bridges, etc
- Soil treatment:In agriculture, quicklime is used to neutralize soil acidity, and it also adds mineral nutrients (Ca2+) to the soil.
- Calcium oxide is dissolved in water to make slaked lime, which is used in the softening of water.
- Drying agent: Calcium oxide is used for drying ammonia and ethanol.
- It is used in the manufacture of bleaching powder, CaOCl2.
- Manufacture of glass: heating a mixture of sand, sodium carbonate and lime or limestone gives glass.
- Lining of furnaces: It is mixed with magnesium oxide to form the basic lining of the furnaces to remove acidic impurities in the form of slag.
- it is used in the blast furnace to remove impurities fromiron ore which is removedin the formof slag.
- Preparation calcium carbide: Calcium carbide (CaC2) is manufactured in an electric furnace at 2000oC. CaO(s)+ 3C(s)→ CaC2(s)+ CO(g)
Uses of magnesium oxide (MgO)
- The oxide is used as a lining material in refractory furnaces, owing its high melting point, which is around 2900oC. It is also used as a refractory agent in the construction of crucibles.
- The oxide in its solution form (magnesium hydroxide) is commonly used as an antacid. This works because magnesium hydroxide is a basic substance, which means that, it will neutralize excess acidity and end up indigestion, caused by too much hydrochloric acid in the stomach.
- It is used to manufacture the common chemical reagents in the laboratory such a magnesium chloride, magnesium sulphate and magnesium hydroxide.
- Magnesium oxide is a popular drying agent. In its powder form, it is hydroscopic in nature. This makes it suitable for drying different substances.
- Insulation: Due to its heat resistance properties, magnesium oxide powder makes an excellent insulator.
- Dietary supplement: Since it is a good source of magnesium, the oxide is used as or in dietary supplements forhumans and animals.
Uses of aluminium (III) oxide (Al2O3)
- Aluminium (III) oxide, in the form of bauxite, is used as a source of aluminium.
- Owing its rough surface, the oxide is used as an abrasive, i.e. it is used to rub and clean other surfaces.
- It is used as an adsorbent in chromatography.
- It is used in the lining of furnaces as a refractory material because it has a high melting point (2040oC).
Uses of zinc oxide (ZnO)
- Zinc oxide is chiefly used in the manufacture of paints and pigments. In addition, the oxide is used to manufacture anti-corrosive coatings, lubricants, adhesive batteries, fire retardants, plastic, cement, glass and ceramics (as a component of glazes).
- Manufacture of rubber: It is mainly used to activate vulcanization, which aims at improving the strength and elasticity of rubber.
- Manufacture of cigarette filter: As a cigarette filter, zinc oxide helps to remove certain harmful compounds from the tobacco smoke, without altering its flavour.
- Making concrete: It helps to make the concrete more resistant to water, besides improving the processing time required.
- Medical uses: Zinc oxide has anti-bacterial properties, for which it is extensively used to treat a number of skin conditions. It is topically applied to provide relief in skin irritation, diaper rash, minor burns and cuts, and for dry and chapped skin. It is added to baby powder, anti-dandruff shampoos as well as antiseptic creams and surgical tapes due to its medicinal properties. In addition, together with iron oxide, it is used to make calamine solution.
- Cosmetic uses: the most important use of zinc oxide in the cosmetic industry is in the preparation of sunscreen lotions and creams. Zinc oxide can absorb ultraviolet (UV) radiation of the sun and thereby protect the skin from sunburn and other damaging effects of UV radiation.
Hydroxides
Carbonates and Hydrogen Carbonates
Nitrates
Chlorides
Sulphates