Join Our Groups
TOPIC 6: STRUCTURE AND PROPERTIES OF MATTER
Structure of Matter
State of matter is defined in terms of the phase transitions which indicate the change in structure and properties. Solids, liquids and gases all are made up of microscopic particles. The behavior of all these particles also varies in three phases.
The Concept of Matter
Explain the concept of matter
Matter is anything that has mass and occupies space. In other words, matter is anything that has weight and volume. For example, stone is considered a matter because it has mass and it occupies space. Therefore, everything we see around us is considered a matter.
The Particular Nature of Matter
Justify the particulate nature of matter
Matter is made up of tiny particles. These particles are either atoms or molecules. That is to say atoms or molecules combine in millions to form matter. Atoms or molecules are combined in chemical reactions to form matter.
Atom is a smallest part of an element that can take part in a chemical reaction. When two or more atoms combine they form a molecule.
The nuclear attractive forces are responsible for holding atoms together in a matter. Some matters have strong attractive nuclear forces between atoms e. g gold, silver, iron. Some matters have weak attractive forces between atoms e. g water, oxygen gas.
The Kinetic Theory of Matter
Explain the kinetic theory of matter
The Kinetic Theory of matter describes the physical properties of matter in terms of the behaviour of its component atom or molecules. It states that
“All matter is made up of very small particles that are in constant motion”
This theory tells us about two important things;
- That matter is a combination of tiny (very small) particles.
- And all these tiny particles are always in motion.
The motion of the particles in a matter was experimented by a scientist known as Robert Brown in an Brownian motion experiment.
Brownian motion
This was an experiment conducted by a scientist Robert Brown to investigate the motion of the tiny particles in a matter.
In his experiment, he put pollen grains in water and observed them using a microscope. He observed that, pollen grains were moving in a random or irregular motion.

Brownian movement
Mr. Brown discovered that, the random motion of the pollen grains in water was caused by the collisions between them and the molecules of water. The pollen grains continued with the random motions since the collisions did not stop.This motion is called Brownian motion.
Brownian motion is an irregular motion of tiny particles suspended in a fluid.
Three States of Matter
Classify three states of matter
There are three states of matter, namely:
- Solid state
- Liquid state
- Gaseous state
Solid state is the state of matter, which include materials in which the inter-molecular force between molecules are greatest and distance between molecules is small. Solids have definite shape and volume. Particles in solid are closely packed together. Examples of solids are wood, iron, glass, rubber, concrete etc.
Liquid state includes substances in which the inter-molecular forces are low compared to solid state, there is greater distance between one molecule and another. Solid shave no defined shape or volume but they take the shape and of the container or vessel. Examples of liquid substances are water, honey, spirit, kerosene, and petroleum.
Gaseous state is the state of matter in which there is no intermolecular forces between molecules hence molecules are free to move from one place to another. Gases have no defined shape or volume but they fill the whole container in which they are kept. Particles in gases are most free compared to solids and liquids. Examples of gases are hydrogen, oxygen, carbon dioxide gas.
Difference between solid state, liquid state and gaseous state of matter
| Solid state | Liquid state | Gaseous state. |
| It concerns with solid matter | It concerns with liquids/ fluids matter | It concerns with gases |
| Have high intermolecular | Low intermolecular force | No intermolecular force |
| No distance between molecules | There is little distance between molecules | Molecules are far from each other |
| Good examples are iron materials, woods etc. | Good examples are water, soda, kerosene and petrol | Good examples are oxygen and hydrogen |
Elasticity
The Concept of Elasticity
Explain the concept of elasticity
Elasticity is the property of a body to return to its original shape and size when deforming force is removed. When force is applied on a body, shape and size may change. If the force is removed away from the body, elasticity will make that body to return to its original shape and size.
All objects that can retain their original shape and size after the deforming force has been removed are known as elastic objects. Example of elastic objects are springs and rubber bands.
Materials which break when force is applied to them are known as brittle materials. Brittle materials do not undergo elasticity. Example of brittle materials is glass.
Materials which do not return to original shape and size after removing the deforming force are known as plastic materials. Plastic materials undergo plastic deformation when extra force is applied. Example of plastic materials are plastic bags, plastic utensils and many more.
The Relationship between Tension and Extension of a Loaded Elastic Material
Justify the relationship between tension and extension of a loaded elastic material
The relationship between tension and extension of a loaded elastic material is well explained in Hooke’s law. It states that
“Within the elastic limit, extension is directly proportional to the force applied (tension)”
Tension can be described as the force(F) transmitted within a string or rope or wire when it is stretched or elongated. Extension (e) is an excess length obtained after stretching a wire or rope or string.
Hooke’s law describes that when a force is applied to an object, the length of an object will increase in the same proportion as the force. If the limit of extension (elastic limit) is not reached the object can return to its original shape and size after the removal of the applied force. However, if the elastic limit is reached then the object will not return to its original shape and size even after removing the applied force.
Hooke’s law can be expressed mathematically as,
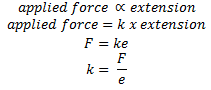
Where ‘k’ is the constant known as force constant or spring constant and its SI unit is Newton per metre (N/m).
The relationship between tension and extension of a loaded elastic material can also be explained using the following graph; -
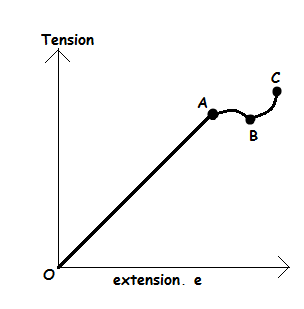
The above graph can be interpreted by describing its parts as follows; -
- Part O – A: Tension is direct proportional to extension. This was discovered by Hooke and final he comes with law which called Hooke’s law. At this stage, the object can return to its original shape and size if tension is removed.
- Point A: This is known as the limit of proportionality or elastic limit.
- Part A – B: This region is called the region of elastic. In this region, a small force produces large extension which is not directly proportional to the extension.
- Part B – C: This region is called the region of plastic deformation. At this region material will not return to its original shape and size when applied force/tension/load is removed.
- Beyond C: Beyond this point the object becomes thinner and ultimately break due to excessive application of force.
The Application of Elasticity in Real Life
Identify the applications of elasticity in real life
In everyday life we often actually do the activities that are concerned with the application of physics. Here are some of the application of physics in everyday life especially in the application of Elasticity:
- Spring mattress. When you sit or sleep on a spring mattress, futon style push your weight. Pressured by the compressed spring mattress. Due to the nature of its elasticity, stretch a spring mattress again. Spring will be stretched and compressed, and so on.
- Spring that is used as shock absorbers on motorcycles. Springs used in the suspension systems of motor vehicles. The purpose of this is to dampen spring a surprise when a motorcycle driven through an uneven road surface.
- Another simple example and that you may often come across is the catapult. When it was about to shoot birds with catapults for example, rubber slingshots first stretch (given the gravity). Due to the nature of its elasticity, long rubber slingshots will return to normal after a tensile force is removed.
Adhesion and Cohesion
The Concept of Adhesion and Cohesion
Explain the concept adhesion and cohesion
Matter is made up of molecules. That exerts force of attraction. This force of attraction may be either Cohesion or Adhesion.
- Cohesion is the force of attraction between the molecules of the same substance, example water to water molecules.
- Adhesion is the force of attraction between the molecule of different substances example water to glass molecules.
Water molecules can experience the force of cohesion among themselves, where water molecules and glass molecules will experience force of adhesion.
Definite shapes of a solid are due to strong cohesion force among its molecules.
Shapes and meniscus of a liquid
When we carried out activities involving determination of volume in a liquid ring and measuring cylinder. The description indicated that the surface of the liquid was carved, forming a meniscus, and that the volume must be read at the bottom or top of the meniscus, depending on the liquid used. For mercury, the top of the meniscus is read.
The formation of a meniscus in a liquid is due to forces of adhesion between the liquid and the walls of the container. The adhesion of the liquid such as water to the wall of a vessel causes an upward force on the liquid at the edge.
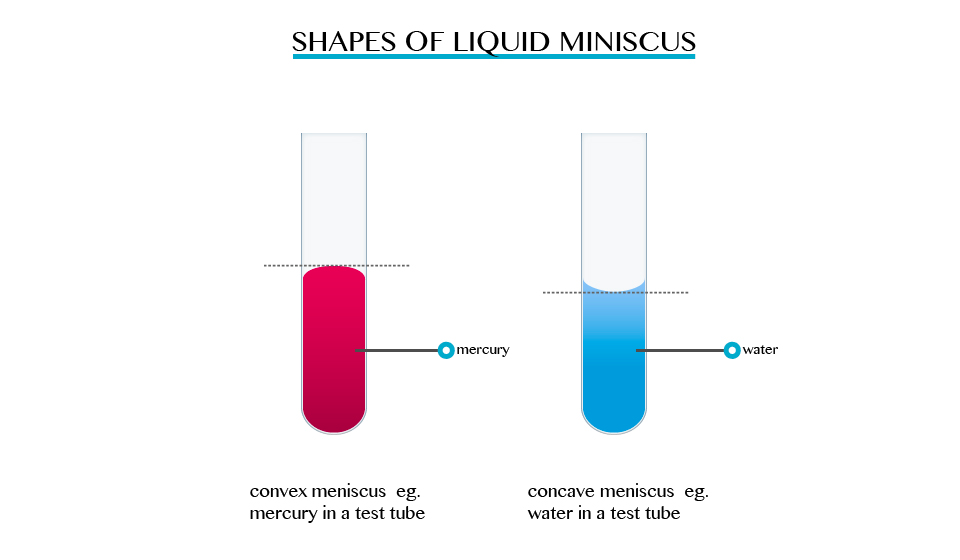
The opposite takes place in mercury, the meniscus of water curves upwards forming a concave shape. When a drop of each liquid, mercury and water are placed on a glass sheet, water spreads further unlike mercury, because of mercury’s high cohesion force among its particle.
Why water wets the glass?
Why methanol does not wet the glass?
Applications of Adhesion and Cohesion in Daily Life
Identify the applications of adhesion and cohesion in daily life
Application include the following
- To stick two different objects together. Here we use the adhesive effects of tape or glue.
- Adhesion can also be used to remove harmful materials such as bacteria from drinking water. Adhesive forces are the source attraction substance.
- Cohesion assists in transport of water in plants and animals by allowing one molecule to pull others along with it.
- The bodies of plants and animals also use the cohesion of tissue to repair damage.
- Ink sticks on paper because of adhesive force between the paper and ink.
Surface Tension
The Concept of Surface Tension
Explain the concept of surface tension
While you may not be able to walk on water, water stride does. This is due to the property of liquid, which is known as surface tension.
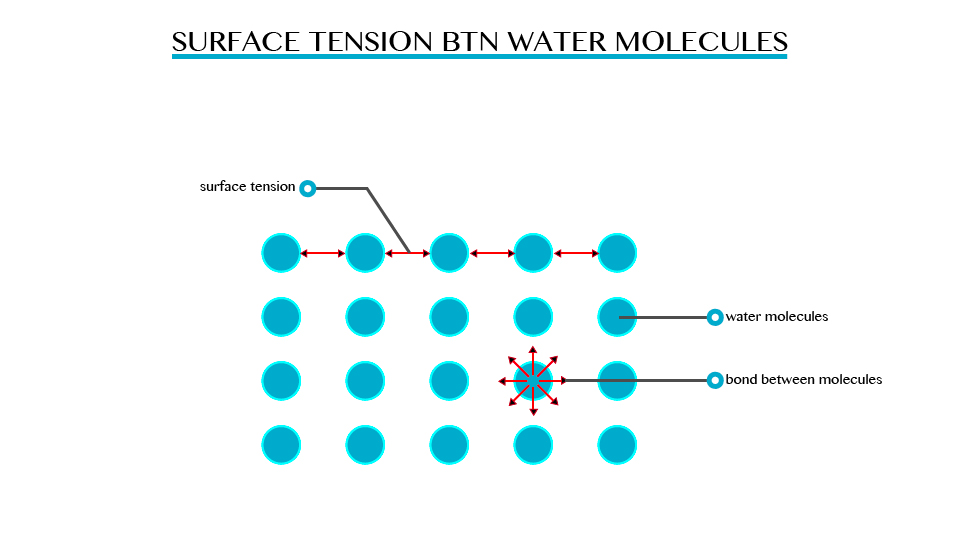
Surface tension is the ability of the molecules on the surface of a liquid to attract and stick to each other allowing them to resist an external force. Surface tension enables insects such as water strides and mosquitoes to walk on water. It allows small objects even metallic ones such as needles and razor blades to float on the surface of water.
Surface tension is a resultant attractive force between molecules in a liquid. The molecules below the surface liquid have forces of attraction between neighbouring particles. However molecules at the surface have no neighbouring molecules above them. This makes them have stronger attractive force than their nearest neighbours on the surface.
However, when some detergent is added to water, the same objects sink to the bottom of the trough. This means that the detergent interfered with the surface of the liquid so decreasing the tension of the water surface.
Detergents are example of surfactants. A surfactant is a substance that reduces the surface tension of a liquid.
Note: the term surfactant is an aerogun for surface-active agent.
Surface tension is affected by the following
- Nature of the liquid
- Contamination/impurities
- Temperature
Application at surface tension:
- In extraction of impurities dating laboratory process.
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap.
Applications of Surface Tension in Daily Life
Identify the applications of surface tension in daily life
Application at surface tension
- In extraction of impurities dating laboratory process
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap
Capillarity
The Concept of Capillarity
Explain the concept of capillarity
This is the tendency of a liquid to rise in narrow tubes or to be drawn into small openings such as those between the fibres of a towel. Capillarity can pull a column of liquid upward until the weight of liquid becomes greater than the surface tension.
In a tube, capillarity depends on the tube’s diameter but weight of water column depends on other factors besides it.
The smaller the radius of the tube the higher the liquid will rise in it. This implies that capillarity height is immensely proportional to the diameter of the tube.
By definition
Capillarity is defined as the tendency of liquid to rise in narrow tubes or to be drawn into small openings such as those between the fibres of a towel.
Capillarity action is the ability of a liquid to raise or fall in a narrow tube.
Note:
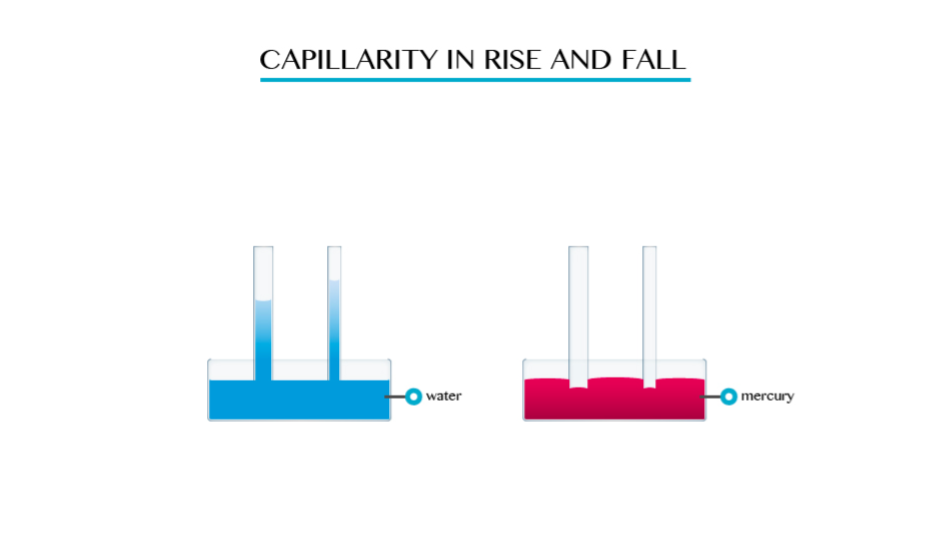
- Capillarity depends on the type of liquid. For example if you dip capillarity tube in water the water rises in the tube and above the level of the water in the vessel.
- If the tube is dipped in mercury, the liquid does not rise in the tube. It suffers capillarity depression.
Applications of Capillarity in Daily Life
Identify the applications of capillarity in daily life
The application includes:
- Capillarity is essential to plants and animals.
- In plants, it facilitates the transport of water and nutrients from the roots to the leaves where photosynthesis produces the plants food. In animals it assists in the circulation of blood.
- Capillarity promotes the movement of ground water.
- It is the principles on which paper and fabric towels work to absorb water.
- Cotton clothing in hot climates uses capillarity action to draw perspiration away from the body.
- In an oil or kerosene lamp capillarity draws the fuel up into the wicker where it can be burnt.
- A writing Rubin splits in the middle so that a fine capillary is formed.
Osmosis
The Concept of Osmosis
Explain the concept of osmosis
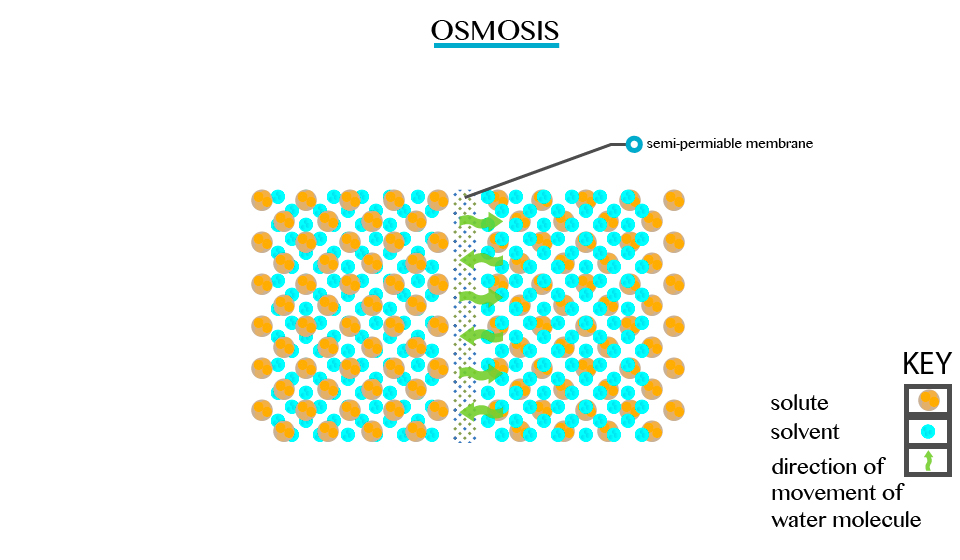
Defined as the movement of a solvent from a region of low concentration through semi permeable membrane.
Particles will diffuse through the membrane in an attempt to equalize the concentration on either side. E.g. two solutions of different concentration separated by a semi permeable membrane. The membrane is permeable to the smaller solvent molecules but not to the larger solute molecules. Osmosis stops when the concentration becomes the same on either side of the membrane.
Osmosis stops when the concentration becomes the same on either side of the membrane.
Applications of Osmosis in Daily Life
Identify the applications of osmosis in daily life
Applications of osmosis in daily life:
- Control the movement of water and nutrients in and out of the cell.
- Filtration processes.
- Removal of harmful ingredients from dinking water.
- Removing salt from seawater so as to make it suitable for drinking and for other domestic uses.





EmoticonEmoticon