JOIN US WHATSAPP
CLICK HERE
JOIN US TELEGRAM
CLICK HERE
TOPIC 5: THERMAL EXPANSION
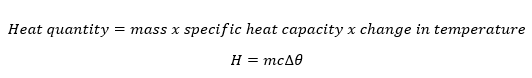
Thermal Energy
The Concept of Heat
Explain the concept of heat
Heat energy (thermal energy) is the form of energy that causes the vibration of the particles of a substance. The more the vibrations the more the heat energy contained.
Changes in the amount of heat energy contained in the substance cause changes in the temperature of the substance.
Temperature – Is the degree of hotness or coldness of a body.
SI Unit of Heat is Joule (J)
The amount of heat content depends on the mass of the body, specific heat capacity and the change in temperature and is mathematically obtained as;
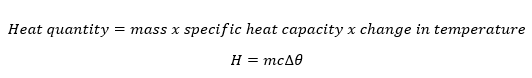
Source of Thermal Energy in Everyday Life
State the source of thermal energy in everyday life
There are numerous sources of energy such as the sun, fuels, nuclear sources, geothermal, electricity among others. The most important source of thermal energy is the sun. The sun generates its energy by a process called thermonuclear fusion. Most sources of thermal energy derive their energy from the sun. Thermal energy from the sun makes life on earth possible.
Difference between Heat and Temperature
Distinguish between heat and temperature
Heat and temperature are related and often confused. More heat usually means a higher temperature.
Heatis energy. It is the total amount of energy (both kinetic and potential) possessed by the molecules in a piece of matter. Heat is measured in Joules.
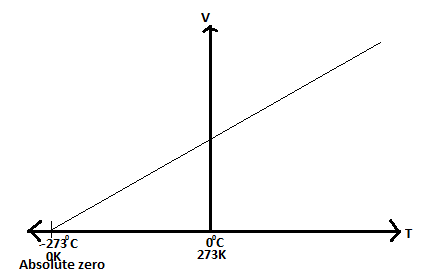
Temperatureis not energy. It relates to the average (kinetic) energy of microscopic motions of a single particle in the system per degree of freedom. It is measured in Kelvin (K), Celsius (C) or Fahrenheit (F).
When you heat a substance, either of two things can happen: the temperature of the substance can rise or the state of substance can change.
| Heat | Temperature | |
|---|---|---|
| Definition | Heat is energy that is transferred from one body to another as the result of a difference in temperature. | Temperature is a measure of hotness or coldness expressed in terms of any of several arbitrary scales like Celsius and Fahrenheit. |
| Symbol | Q | T |
| Unit | Joules | Kelvin, Celsius or Fahrenheit |
| SI unit | Joule | Kelvin |
| Particles | Heat is a measure of how many atoms there are in a substance multiplied by how much energy each atom possesses. | Temperature is related to how fast the atoms within a substance are moving. The ‘temperature’ of an object is like the water level – it determines the direction in which ‘heat’ will flow. |
| Ability to do work | Heat has the ability to do work. | Temperature can only be used to measure the degree of hea |
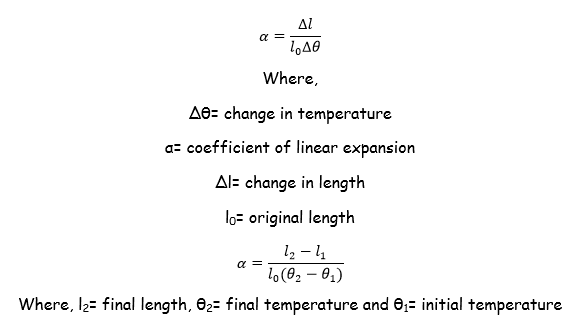
Thermal Expansion of Solids
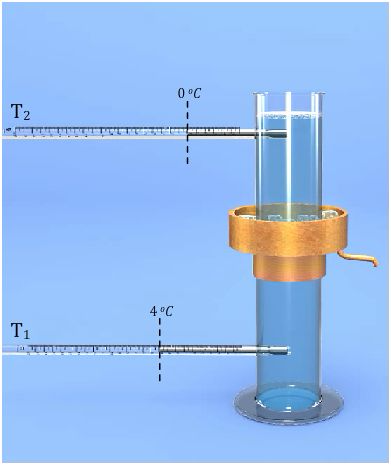
Thermal Expansion of Liquids
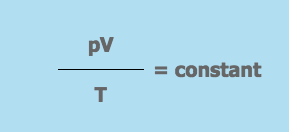
Thermal Expansion of Gases






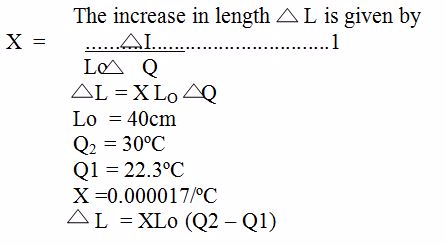
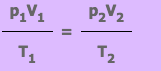

1 Comment
Exellent