JOIN US WHATSAPP
CLICK HERE
JOIN US TELEGRAM
CLICK HERE
TOPIC 7: MEASUREMENT OF THERMAL ENERGY
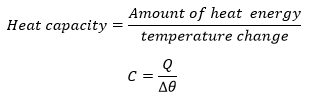
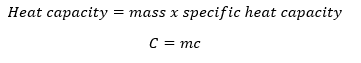
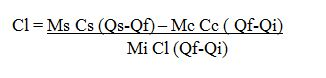
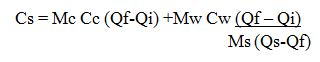
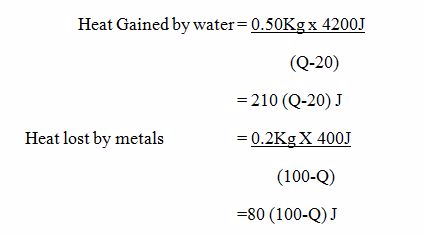
Heat Capacity
Heat capacity is the amount of heat required to raise the temperature of an object or substance by one degree. The temperature change is the difference between the final temperature ( Tf) and the initial temperature ( Ti).
The Factors which Determine Heat Quality of a Substance
Explain the factors which determine heat quality of a substance
Heat is a form of energy transferred between bodies due to difference in temperature between them. The energy possessed by the body due to its temperature is called the internal thermal energy. The heat content is due to the random motion of the particles that make up the body. The heat content of the substance depends on;
- mass of the substance
- temperature change
- specific heat capacity of the substance
The Heat Capacity
Determine the heat capacity
Heat capacity is the quantity of heat required to raise the temperature of an object by one degree Celsius.
Different objects have different heat capacities. That means, some can feel so hot than others for the same amount of heat energy supplied to them. For example, hot food on a metal plate feels very hot and difficult to touch than hot food on a plastic plate because a metal plate absorbs heat and raises its temperature faster than plastic plate. In this case we can say plastics have higher heat capacity than metals. In other words, it requires small amount of heat energy to raise temperature of metals than than in plastics.
If the object requires amount/quantity of heat energy Q to raise its temperature by one degree of centigrade, then the heat capacity C of the substance is obtained as;
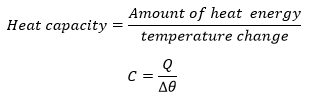
Heat capacity can also be obtained mathematically by taking the product of mass of the substance and its specific heat capacity.
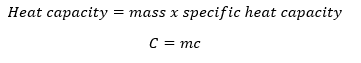
Example 1
Find the heat capacity of a lump of copper of mass 50kg. The specific heat capacity of copper is 420 J/ Kg ºc.
Data Given
Mass of copper, m= 50kg
The specific heat capacity of copper, c = 420J/KgºC
Required: To calculate heat capacity, C.
C = mc
= 50Kg x 420J/KgºC
= 21600J
=21KJ
Calculating a quantity of heat
- The quantity of heat required to change the temperature of a body with mass, mkg byθ degree Celsius is mcθ joules.
- In order to raise the temperature of a body, heat must be supplied to it.
- In order to lower its temperature, heat must be removed from it.
The Heat Equation is therefore written:
Heat Gained or Heat Lost = Mass X specific heat capacity X change in temperature
Change in temperature:
H =mcθ
Where
H=Heat gained / lost
m= Mass of the body
θ= change (Rise or fall) In Temperature of the body.
Example 2
Water of mass 3kg is heated from 26ºc to 96ºC. Find the amount of heat supplied to the water given that the specific heat capacity of water is 4.2 x 103 J / Kg ºc
Data Given
Mass of water, m = 3kg
Specific Heat capacity, c = 4.2 X 103 j / kgº C
Initial temperature, θi = 26 ºC
Final Temperature, θf = 96ºC
Required
The amount of heat, H
H= mcθ
c = H/mθ
H = mcθ
H = 3Kg x 4.2 x103 (96-26) ºC
The amount of heat energy is H= 882000J= 882KJ
The Specific Heat Capacity
Determine the specific heat capacity
Specific heat capacity is the quantity of heat required to raise the temperature of a unit mass of a substance by one degree Celsius.
The quantity of heat supplied to or taken away from a body depends on:
- The mass of the body, M
- The temperature different, ΔT
- The thermal properties of the body.
Transfer of Heat
Heat energy tends to flow from High temperatures to Low temperatures
If you pick up a warm object, heat energy transfers from the object to your hands and your hands feel warm. If you pick up a cool object, heat energy transfers from hands to the object and your hands feel cold.
Determining specific Heat capacity
- Calorimeter – Is the special instrument or vessel used for measurement of Heat.
- Calorimeter is highly polished metal can usually made of copper or aluminium.
- It is flitted with an insulating cover in which there are two holes.
- Two holes allow a thermometer and a stirrer to be inserted.
- The stirrer is made of the same metal as that of the calorimeter.

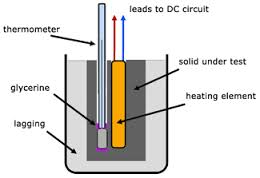
Demonstration of the specific Heat capacity of a solid
Determining specific Heat capacity by Method of Calculation.
Heat lost by solid, Hs = Ms x Cs (Qs – Qf)
Heat Gained by Calorimeterand stirrer, Hc = Mc x Cc (Qf – Qi)
Heat Gained by Water, HW = Mw x Cw (Qf – Qi)
But the heat lost by the solid is equal to heat gained by the calorimeter and stirrer plus the heat gained by the water in the calorimeter.
Hs = Hc + Hw
But
Heat gained by a calorimeter and content equal to heat lost by the solid.
Thus Hc + Hl = Hs
Mc Cc (Qf – Qi) + Mi Ci ( Qf-Qi) = Ms Cs ( Qs-Qs)
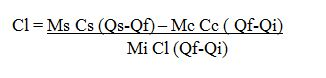
Example 3
A piece of metal with a mass of 200g at a temperature of 100ºC is quickly transferred into 50g of water at 20ºC find the final temperature of the system ( specific Heat capacity of water Cw = 4200J/ Kg ºC specific Heat capacity of the metal Cm = 400J/KgºC.
Ms Cs (Qs-Qf) = Mc Cc (Qf-Q) Mm Cw (Qf-Ql)
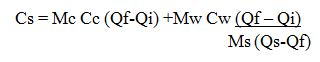
Where:
Cs. Is the specific Heat capacity of the solids.
Determining the specific heat capacity of liquid, Cl
By calculation method;
Heat Gained by calorimeter and stirrer
Hc = Mc Cc (Qf – Qi)
Heat Gained by liquid
Hi = ML CL (Qf – Qi)
Heat lost by the solid
Hs = Ms Cs ( Qs-Qf)
Let
Q be the final Temperature of the system
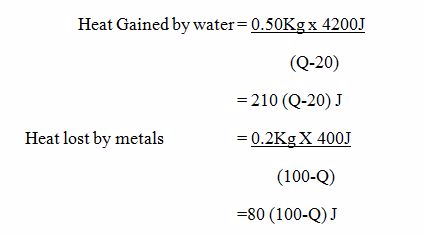
If there are no heat Losses to the surroundings, then.
(Heat gained by water) = (Heat lost by metal)
210 (Q-20) = 80(100 – Q)
21(Q-20) = 8 (100-Q)
21Q – 420 = 800 – 8Q
21Q +8Q = (800+420)
29Q = 1220
Q = (1220/29)
Q = 42.1ºC
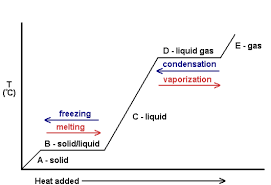
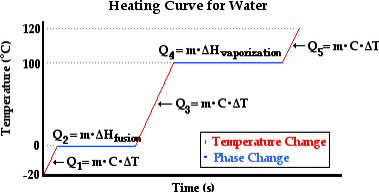
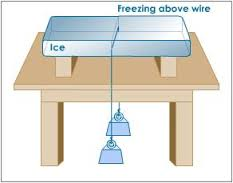
Change of State