TOPIC 6: TRANSFER OF THERMAL ENERGY

Conduction
The Concept of Conduction of Heat
Explain the concept of conduction of Heat
Conduction is the transfer of heat energy through solids in the manner which particles do not move from one point to another, for example in metals. Generally, solid substances contain particles which are close together. Each particle vibrates at one position but cannot move to another position.
The heated particles vibrate and collide with the other particles adjacent to them. In this way heat is transferred to the adjacent particles. The process goes on until the whole body get heated.
Good and Bad Conductors of Heat
Identify good and bad Conductors of Heat
Solid materials differ greatly in their ability to conduct HEAT.
Good conductors
These are the substances which allows the passage of heat energy easily example all metals.
Metals contain tiny particles called electrons (particles that carry electricity through metals) which are free to move inside the metal and carry energy from hotter places to colder places.
Bad conductors
These are materials which does not allow the passage of heat and electricity e.g Non – metals, woods.
| GOOD CONDUCTOR | BAD CONDUCTOR |
|
|
How to Minimize Heat Losses due to Conduction
Explain how to minimise Heat losses due to Conduction
There are some simple ways to reduce heat loss, including fitting carpets, curtains and draught excluders.
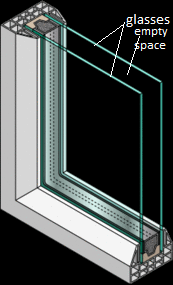
Heat loss through windows can be reduced using double glazing. The gap between the two panes of glass is filled with air. Heat loss through conduction is reduced, as air is a poor conductor of heat. Heat transfer by convection currents is also reduced by making the gap is very narrow.

A double glazed wall
Heat loss through walls can be reduced using cavity wall insulation. This involves blowing insulating an material into the gap between the brick and the inside wall, which reduces the heat loss by conduction. The material also prevents air circulating inside the cavity, therefore reducing heat loss by convection.

Cavity wall insulation
Knowledge of Conduction in Daily Life
Apply knowledge of conduction in daily life
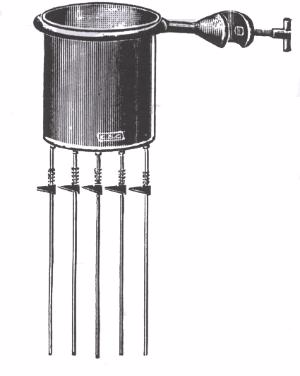
The difference in conductivity of various materials can be demonstrated using Edser’s apparatus

The apparatus consists of copper can with identical rods of aluminum, copper, lead and iron fixed to the bottom of the can. These identical rods are smeared/coated with wax.
The can is supported by a metal ring which is clamped to a retort stand. When hot water is poured inside the copper can, heat will be passed along the rods by conduction.
After some time, it will be observed that wax coated on the rods will melt and move down the rods. Note how far along the rods the wax has melted when the apparatus reaches a steady state.
This indicates that the materials from which the rods are made have different thermal conductivities. Of the four metal rods, the copper rod is observed to conduct heat more quickly than the rest.
Conduction of Heat Energy through Liquids
- All liquids expect mercury and gases are poor conductors of heat.
- Gases are far worse conductors of heat than liquids.
- Fluids are bad conductors of heat. They transfer heat by means of convection.
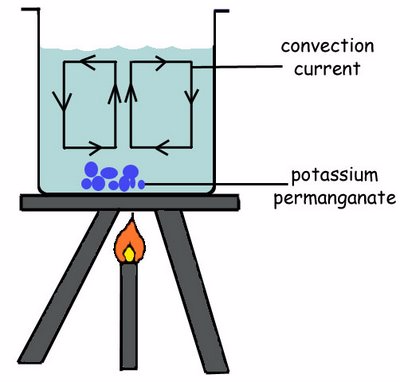
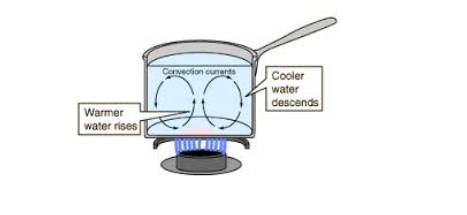
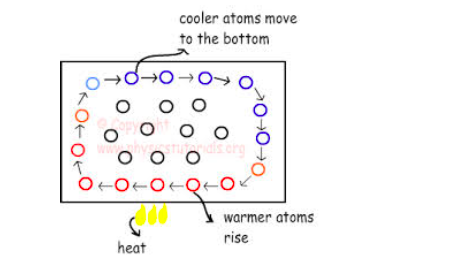
Convection
Radiation