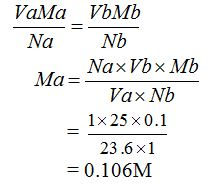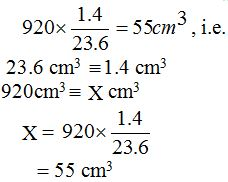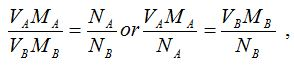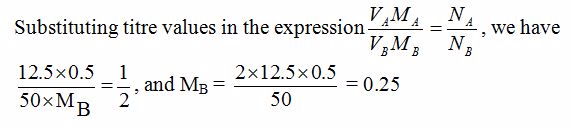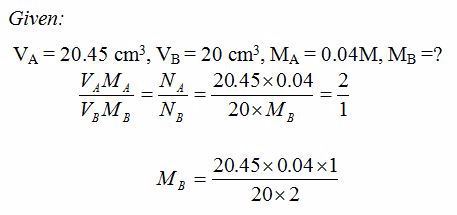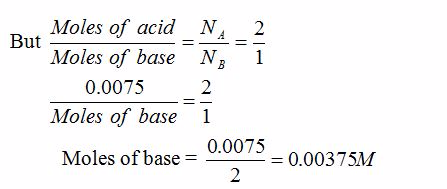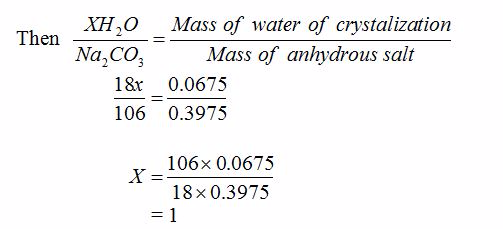TOPIC 5: VOLUMETRIC ANALYSIS

Standard Volumetric Apparatus
The Concept of Volumetric Analysis
Explain the concept of volumetric analysis
Volumetric analysis is a quantitative analysis involving the measurement of different solutions. These solutions are made to react completely and the completion of the reaction is indicated by certain substances called indicators. The quantitative composition of the solution is then determined.
Important steps of volumetric analysis include:
- Weighing;
- Preparation of the solution;
- Titration; and
- Calculation
In volumetric analysis, we deal with volumes of solutions. That is why this quantitative determination of solutions of substances is called volumetric analysis.
The amount of a substance present in a solution is given in terms of its volume and its concentration. The volume of a solution is usually given in litres (dm3). The concentration of a solution is given in moles per litre (mol/dm3) or grams per litre (g/dm3).
Volumetric analysis is a means of finding the concentration of an unknown solution. For example, the concentration of an unknown solution of an acid can be found if it is reacted with a standard solution of an alkali. A standard solution is one whose concentration is well known and does not change with time.
In volumetric analysis, the reaction is carried out in a carefully controlled way. The volumes are measured accurately using a pipette and burette. The method is to add a solution of one reactant to the solution of another reactant until the reaction is complete. When the reaction is complete, we say the end-point has been reached. If the reactants are acids and bases, completion (end-point) is determined by the change in colour of an acid-base indicator. The method is called titration. In other reactions, completion is determined by a colour change of reactant(s). The concentration of one of the reactant solutions must be known in order to be able to find the concentration of unknown solution.
Significance of Volumetric Analysis
- Volumetric analysis is used to quantify the amount of substances present in solutions by analytical procedure, which involves precise measurements of volumes of solutions and masses of solids.
- Volumetric analysis helps in the determination of the accurate volumes and concentrations of the reacting substances, often solutions.
- Volumetric analysis (titration) helps in the preparations of standard solutions.
- Volumetric analysis knowledge helps in the standardization of acids and bases.
Volumetric Apparatus
Use volumetric apparatus
We have seen that volumetric analysis involves determinations of quantities of substances, usually acids and alkalis, present in volumes of solutions. This is usually done by using measuring apparatus.
Apparatus used in volumetric analysis is based on volume measurements and since the analysis demands high accuracy, the apparatus has to be calibrated with the highest possible accuracy. It is for this reason that all apparatus for volumetric analysis are specifically for this and not other purposes.
Apparatus used for volumetric analysis include, burette, pipette, burette stand, white tile, conical flask, filter funnel, reagent bottle, watch glass, beaker, measuring cylinder and measuring flask (or volumetric flask). For approximate measurements, measuring cylinders may be used. For accurate measurements of volumes, volumetric flasks are used.
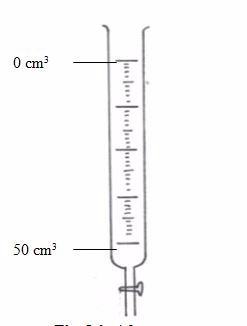
Burette
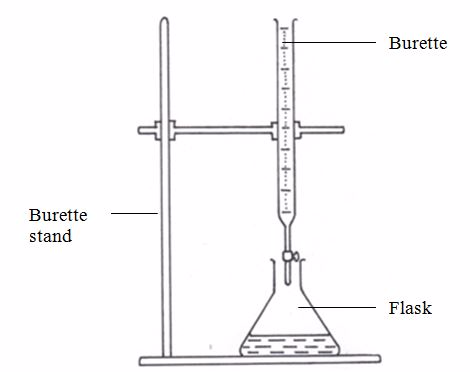
This is a long glass tube with a narrow lower part, which is fitted with a tap that controls the amount of solution let out of the burette. This instrument is calibrated from 0 to 50 cm3.Before measuring the solution, rinse the burette with distilled water, then with the solution it is going to hold. It has to be filled to the tip and all gas bubbles removed. Thus, the burette is an apparatus used for transferring the solution to the titration vessel (normally a flask).

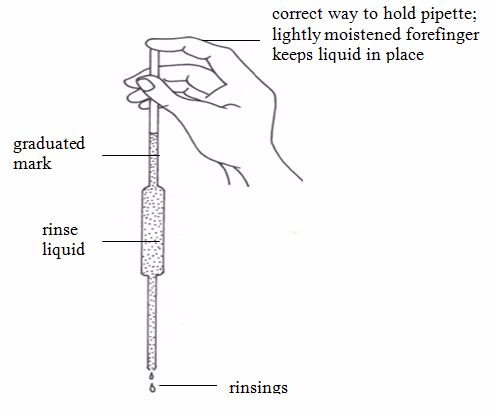
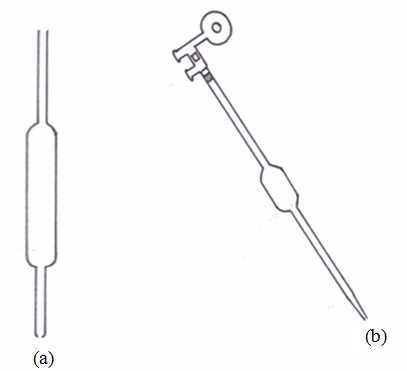
Pipette
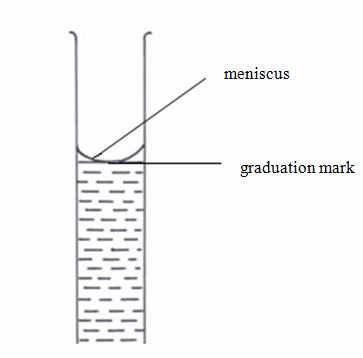
This apparatus has a wider middle part with narrow parts at either ends. The upper narrow part has a mark which marks the volume of all the space below it. If, say, the pipette is one that is marked 25 cm3, we can say that a solution, when filled in the pipette up to this mark, will have a volume of 25 cm3.
The pipette is used in transferring a standard solution to the titration flask. There are many types of pipettes depending on their volume capacity. The common ones are the 25-cm3 and 20-cm3 capacity pipettes. Less common ones are the 10-cm3 capacity.Before measuring the solution, rinse the pipette several times with distilled water and then with the solution to be measured; suck the rinsing solution above the graduated mark, then discard the rinsing.

The pipette is commonly filled by mouth suction but the use of pipette fillers is highly recommended. When using a pipette, never blow out the last drop.

(a) A pipette (b) A pipette and pipette filler (used to fill and empty pipettes)
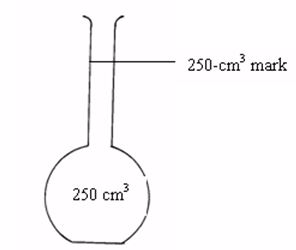
Measuring (Volumetric) flask
The flask is made of glass and has a mark at the upper part of the narrow tube. The space in the flask up to this mark represents a certain volume. If a solution is filled up to this mark, the volume of the solution is equal to the volume indicated by inscriptions on the flask e.g. 50 cm3, 100 cm3, 150 cm3, 250 cm3, 500 cm3, etc.

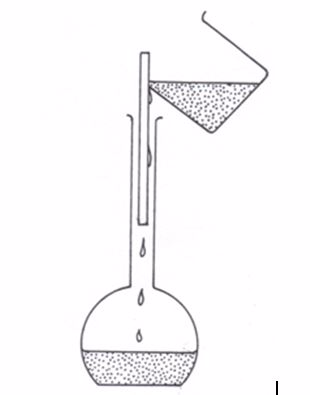
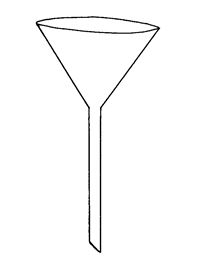
Filter funnel
A filter funnel is required for effective transfer of the weighed solid, liquid or solution into the volumetric flask or burette.

A filter funnel
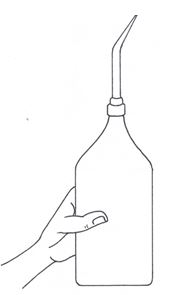
Wash bottle
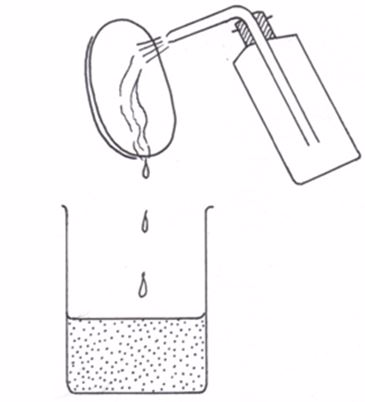
Wash bottle contains water and when squeezed, water squarts out. This is used in washing down the remains of the weighed solid into the volumetric flask.

A wash bottle
A weighing bottle
This is used in weighing the solute. It is a stoppered bottle. A watch glass can also be used to serve the same purpose.
Retort stand
A burette stand is used for holding the burette in place while carrying out volumetric analysis experiments.

A burette stand
Dropper
A dropper is used to add the indicator dropwise into the solution.

White tile or paper
A white tile or piece of paper is placed under the flask to give a clear background for accurate observation of the colour change at the end of the reaction (end point).

Standard Solutions
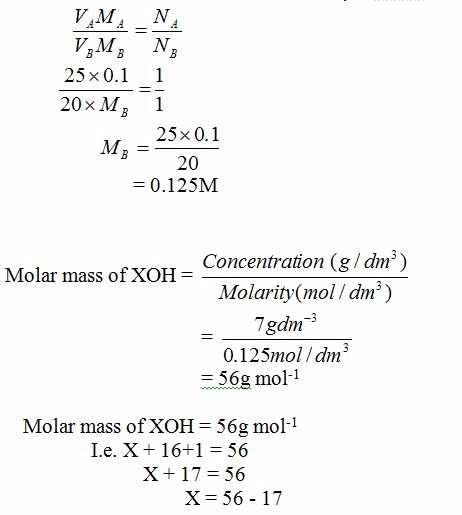
Volumteric Calculations
Application of Volumetric Analysis