TOPIC 2: HARDNESS OF WATER
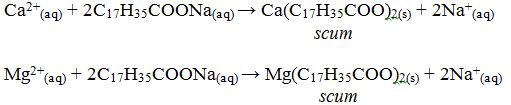
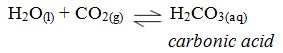
The Concept of Hardness of Water
The Concept of Hardness of Water
Explain the concept of hardness of water
As water flows over the land, it dissolves many mineral substances. The dissolved minerals are deposited together with water in rivers, lakes and oceans. Water is said to be hard if it contains some specific type of dissolved minerals. It is important to note that not all dissolved salts make water hard.
As you learned early, water is treated in water purification plants before being piped to your home. The treatment removes only the insoluble particles and kills bacteria. So the water still is not pure. It contains natural compounds dissolved from rocks and soil. It may also contain traces of chemicals dumped from homes, farms and factories.
Water obtained from an area where the rocks contains chalk, limestone, dolomite or gypsum, contains dissolved calcium and magnesium sulphates and hydrogen carbonates. These salts make the water hard.
One can distinguish between hard and soft water when washing with soap. Hard water does not form lather easily. Instead, it forms a precipitate or scum. It requires much soap to react with all the dissolved minerals before enough lather is formed. Therefore, hard water wastes soap during washing.
When soap is used with hard water a “scum” forms on the surface. This is a result of a precipitation reaction between calcium and/or magnesium ions and soap. Soaps are the sodium or potassium salts of long-chain organic acids. Soaps are made from animal fats by treatment with alkali (NaOH or KOH). Ordinary washing soap is a compound of stearic acid, C17H35COOH. The nature of such soaps is the salt, sodium stearate, C17H35COONa+. Sodium stearate is soluble in water but calcium stearate is not.
When soap is mixed with hard water, the calcium or magnesium salts in the hard water react with soap and precipitates as scum. The nature of scum is either calcium stearate or magnesium stearate:
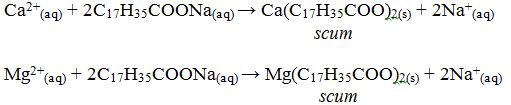
Soap will not form any lather with water until all the calcium and magnesium ions have been precipitated. Hard water, therefore, wastes soap. This means that more soap may be used for an efficient washing. The amount of soap needed to just produce froth can be used to estimate the hardness of water.
The problem of scum formation only occurs with soaps. Soapless detergents do not produce scum. The trade names for some soapy detergents sold in Tanzania include Komoa, Kuku, Taifa, Mbuni, Mshindi, Changu, Jamaa and several other bar soaps. The trade names for some soapless detergents include Omo, Foma, Tesa, Toss, Dynamo, Swan, etc.
Causes of Permanent and Temporary Hardness in Water
State causes of permanent and temporary hardness in water
Water is generally said to be hard if it contains soluble salts of calcium and magnesium. The salts are calcium and magnesium sulphates and hydrogen carbonates. Hardness of water is caused by higher than usual levels of calcium (Ca2+) and magnesium (Mg2+) ions in water.
As rain water passes through the atmosphere, it dissolves carbondioxide to form a weak carbonic acid.
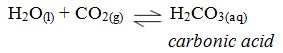
As this solution passes over and through rocks containing chalk, limestone or dolomite, the rainwater very slowly dissolves them:
H2CO3(aq) + CaCO3(s) → Ca(HCO3)2 (aq)
The calcium hydrogen carbonate formed is soluble in water and is responsible for the presence of calcium (Ca2+) ions in water.
Some of the rocks may contain gypsum (CaSO4.2H2O), an hydrite (CaSO4), Kieserite (MgSO4.H2O) or dolomite (CaCO3. MgCO3) which can dissolve to a limited extent in water. The presence of these dissolved substances also causes the water to be hard. These substances dissolve sparingly in water to form Ca2+ and Mg2+ ions which are responsible for water hardness as stated early.
Activity 1
Investigation of the causes of water hardness
Materials:
- Test tube rack-
- Five clean test tubes
- Measuring cylinder (100cm3)
- Calcium sulphate solution (1 mol dm-3)
- Soap solution
- Magnesium sulphate solution (1 mol dm-3)
- Sodium sulphate solution (1 mol dm-3)
- Potassium sulphate solution (1 mol dm-3)
- Distilled water
Procedure:
- Label five clean and dry test tubes as A, B, C, D and E. 2
- Add 10 cm3 of 1.0M calcium, magnesium, sodium and potassium sulphate solutions and distilled water in each of the test tubes respectively.
- Add 5 cm3 of soap in each test tube
- Shake the test tubes well and place them in a test tube rack
- Observe the amount of lather formed in each test tube, and if there is any precipitate (scum) formed.
Results:
Results of experiment showing minerals which cause water hardness
| Test tube | Salt present | Ions present in solution of salt | Lather or scum formed? | Water hard or soft? |
| A | calcium sulphate | Ca2+, SO42- | scum is formed | Hard |
| B | magnesium sulphate | Mg2+ , SO42- | scum is formed | hard |
| C | sodium sulphate | Na+, SO42- | lather is formed | soft |
| D | potassium sulphate | K+, SO42- | lather is formed | soft |
| E | distilled water | no ions | lather is formed | soft |
Interpretation of the results
From the result of experiment, we can conclude that scum is produced when either calcium or magnesium salt is present in water. So, high levels of calcium or magnesium ions in water are responsible for water hardness.
When the concentration of either of these minerals is over 150 milligrams per cubic decimeter (150 mg/dm3), water is considered to be hard. The upper limit allowed is 300 mg/dm3
Types of Hardness of Water
Treatment and Purification of Hard Water


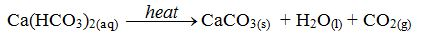
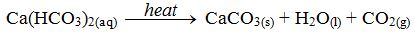
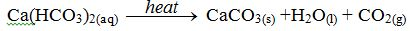
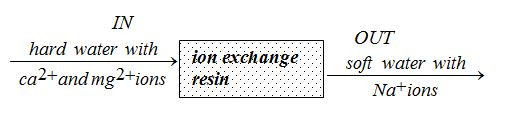

1 Comment
I like the notes